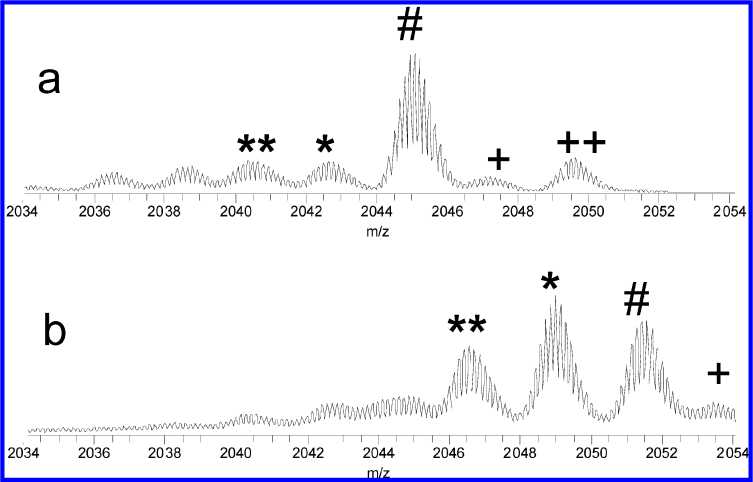
Figure 4. Loss of neutrals from lysozyme ions under ECD. (a) Unmodified and (b) singly nitrated lysozyme. The # symbols label (M + 10H)7+
reduced ions, * and ** label losses of 17 and 34 Da, respectively, and + and ++ label peaks of naturally oxidized protein.
|
Table 2. Number of Cleavages within 6 Amino Acid Residues Both N- and C-Terminal to the Site(s) of | ||
|
Nitration |
ECD |
IRMPD |
|
mononitrated myoglobin |
1 |
2 |
|
mononitrated lysozyme |
2 |
4 |
|
bis-nitrated lysozyme |
2 |
6 |
|
mononitrated cytochrome c |
n/a |
3 |
|
bis-nitrated cytochrome c |
0 |
3 |
cleavages were either not efficient or were not proceeded by
efficient backbone cleavages.
ECD in cytochrome c was hindered by the presence of two
nitro groups and one heme group. While nitro groups act as
electron predators,38,39 the heme group is known to interfere with
the normal course of events during ECD.41 We also note that the
amount of sample of nitrated cytochrome c was much smaller
than that of nitrated myoglobin or lysozyme, because the reaction
of nitration of cytochrome c was less efficient than for the other
two proteins. Consequently, all three MS/MS methods were less
efficient for cytochrome c than for myoglobin and reduced
lysozyme.
CID produced more fragments than IRMPD from unmodified
myoglobin (35 vs 26, Figure S-1 in the Supporting Information).
For all the nitrated proteins studied, CID produced fewer frag-
ments than IRMPD. The reasons for such behavior are not
completely clear. Both MS/MS technique are “slow-heating”
threshold fragmentation methods. IRMPD is often regarded as a
more efficient fragmentation technique, as it acts upon not only
the isolated precursor ions but also upon their primary fragments,
thus producing additional smaller fragment ions. Contrary to that,
the CID waveform is tuned to affect mostly the isolated precursor
ions. Additionally, in our instrument CID is carried out in the linear
ion trap and the fragments transported to and analyzed in the
ICR cell, while IRMPD is carried out directly in the ICR cell.
Transmission of CID fragments to the ICR cell could induce
additional ion losses. Furthermore, in order to provide sufficient
abundance of the fragment ions from top-down MS/MS analysis
of intact proteins, one has to isolate and fragment a much larger
number of precursor ions than in the case of bottom-up MS/MS
analysis of peptides. This is due to a much larger number of
dissociation channels in MS/MS of intact proteins. The number
of precursor ions we typically isolate in the linear ion trap
corresponds to an AGC value of 106-107 for intact proteins, while
for peptide ions AGC values are 1-2 orders of magnitude
smaller. The larger number of intact protein ions in the LTQ
should cause stronger space-charge effects during CID. All CID
measurements presented in this work were taken at the CID
threshold energy. We observed that above the threshold to
CID the precursor protein ions were quickly depleted but that
did not lead to the production of new fragments. This may be
an indication that many of the precursor ions were expelled
from the LTQ during CID, rather than fragmented, or lost
during transportation from the LTQ to the ICR cell for mass
analysis. The effect was greater for CID of nitrated myoglobin
and cytochrome c, where a two or three-step narrow isolation
was used in order to isolate the nitrated protein ions from the
remaining unmodified proteins. A plausible explanation for this
CID behavior is that the space charge effects in the LTQ were
aggravated by the narrow isolation and led to destabilization
of the fragment ion trajectories during collisional excitation.
Both ECD and IRMPD should suffer less from this effect, as
they produce fragments inside the ICR cell and losses of the
fragments there should be minimal.
CONCLUSIONS
Our previous studies demonstrated that the high electron
affinity of the nitro group inhibits normal ECD process in
nitrotyrosine-containing peptides.38 Results from top-down MS/
MS on tyrosine nitration in myoglobin, lysozyme, and cytochrome
c presented in this work indicate that ECD can be used to study
intact nitrated proteins. ECD provided the largest total number
Analytical Chemistry, Vol. 82, No. 17, September 1, 2010 7291
More intriguing information
1. The name is absent2. The name is absent
3. Multifunctionality of Agriculture: An Inquiry Into the Complementarity Between Landscape Preservation and Food Security
4. The storage and use of newborn babies’ blood spot cards: a public consultation
5. Centre for Longitudinal Studies
6. The name is absent
7. The Demand for Specialty-Crop Insurance: Adverse Selection and Moral Hazard
8. Ronald Patterson, Violinist; Brooks Smith, Pianist
9. Strategic Policy Options to Improve Irrigation Water Allocation Efficiency: Analysis on Egypt and Morocco
10. Income Mobility of Owners of Small Businesses when Boundaries between Occupations are Vague