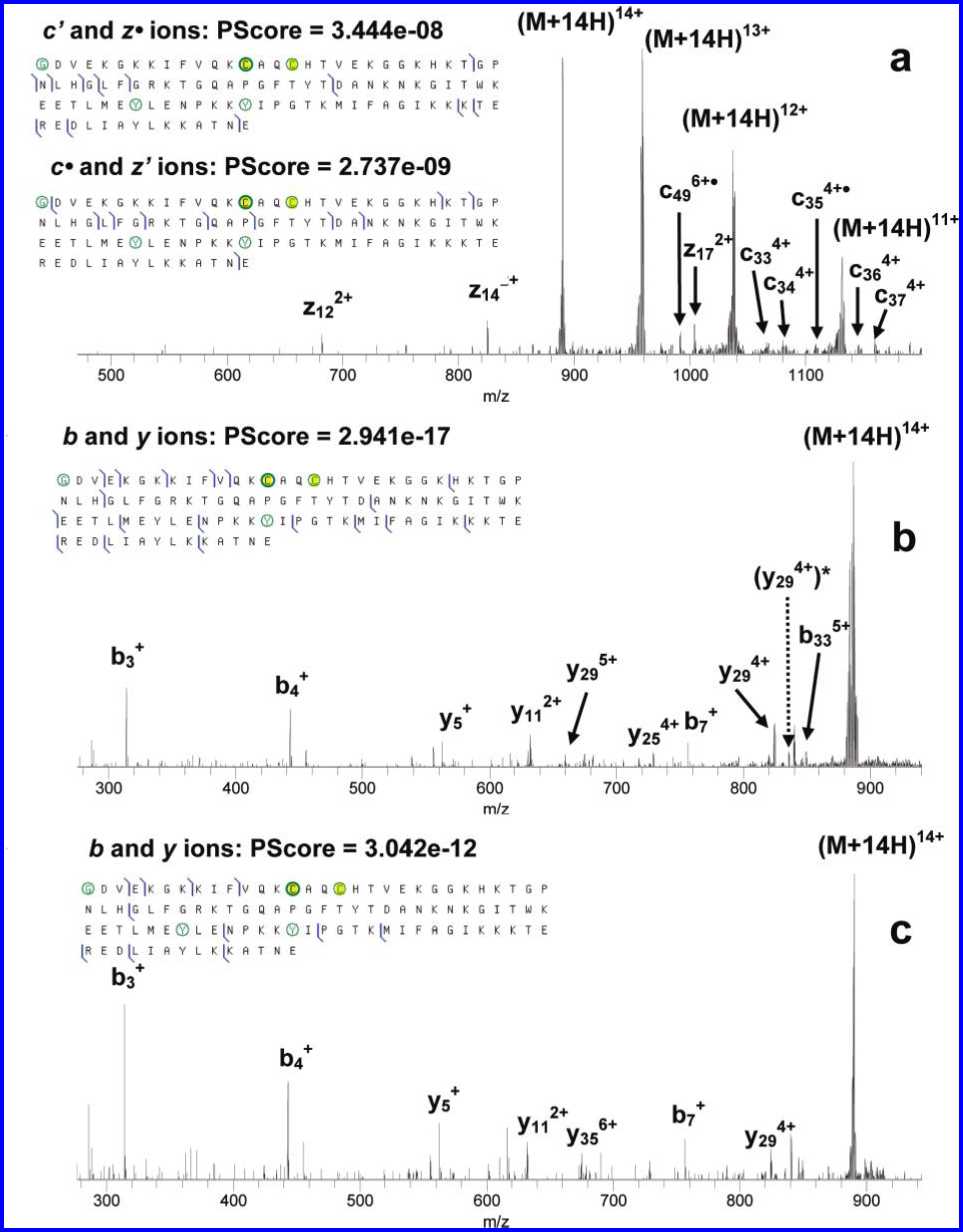
Figure 3. ECD (a) and IRMPD (b, c) of mono- (b) and bis-nitrated (a, c) cytochrome c. N-acetylated glycine, nitrated tyrosines, and cysteines
bound to the heme group are circled.
limited cleavage of the disulfide bonds by ECD. Cleavage of all
four S-S bonds in lysozyme would require the capture of at least
four electrons. We did record up to four consecutive electron
capture events taking place for the 10+ charge state of nitrated
lysozyme, which was indicated by the presence of (M + 10H)6+
charge-reduced ions in the ECD mass spectra. There is a
possibility that both the disulfide bond and backbone cleavages
occurred, but the resulting ECD fragments were held together
by noncovalent interactions in the protein. However post-ECD
IR activation, which we had previously used to release ECD
fragments from the charge-reduced ions,40 did not produce new
fragments. Therefore we conclude that multiple disulfide bond
7290 Analytical Chemistry, Vol. 82, No. 17, September 1, 2010
More intriguing information
1. Three Strikes and You.re Out: Reply to Cooper and Willis2. Transgression et Contestation Dans Ie conte diderotien. Pierre Hartmann Strasbourg
3. The name is absent
4. Life is an Adventure! An agent-based reconciliation of narrative and scientific worldviews
5. The name is absent
6. Return Predictability and Stock Market Crashes in a Simple Rational Expectations Model
7. The Modified- Classroom ObservationScheduletoMeasureIntenticnaCommunication( M-COSMIC): EvaluationofReliabilityandValidity
8. The name is absent
9. The name is absent
10. Optimal Taxation of Capital Income in Models with Endogenous Fertility