Fletcher et al. BMC Cardiovascular Disorders 2010, 10:37
http://www.biomedcentral.com/1471-2261/10/37
Page 6 of 8
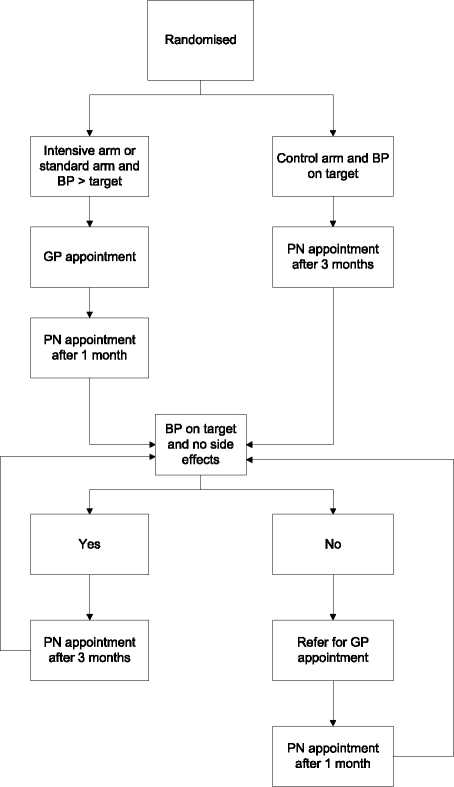
Figure 2 follow up procedure.
the standard deviation in the PROGRESS Study [5]).
The calculation assumes that: a 5 mmHg difference in
systolic BP is of clinical significance, leading to a 20%
reduction in major vascular events;[22] that there will
be 5% mortality at six months, and a further 10% of
patients will not have their BP measured at six months.
With regard to ambulatory blood pressure measure-
ment, one of the secondary outcomes of the study, ran-
domisation of 450 patients (225 per arm) will detect a
4 mmHg difference in systolic BP between groups with
90% power and at a significance level of 5% assuming a
standard deviation of 11.7 mmHg [23]. This calculation
assumes that 80% of patients will have ambulatory
blood pressure measured at 12 months.
To recruit patients from primary care, an estimate of
the number of practices is required. Approximately 50
practices with an average list size of 7,500 will be
needed in order to recruit the required number of
patients. This will generate 5,625 patients on practice
TIA/stroke registers (from the QOF data, the overall
prevalence of TIA/stroke in primary care is 1.5%). From
our analysis of South Birmingham data [11], we antici-
pate that 13% of these patients will be ineligible because
they are already on three or more anti-hypertensives,
and 28% because they will not fulfil the diagnostic cri-
teria for a history of stroke or TIA for the study [24].
We assume that 30% of patients will respond to the
invitation to attend a study clinic, that 24% of them will
be ineligible due to a systolic BP below 125, and a
further 15% will decline to take part after discussion
with the research nurse. This equates to the recruitment
of 12 patients per practice with an average list size of
7,500.
Statistical analysis
The principal analyses will use generalised linear mod-
els, accounting for baseline BP as a patient level covari-
ate, and practices as random effects and compare
differences in systolic BP (primary outcome), and differ-
ences in diastolic BP, quality of life, adherence and fre-
quency of adverse effects (secondary outcomes). We will
look at effect on systolic BP lowering in pre-specified
sub-groups: diabetes; atrial fibrillation; and age group.
Clinical event rates will be monitored by treatment allo-
cation by the Data Monitoring & Ethics Committee, but
only aggregated rates will be made available to the
investigators.
Economic evaluation
Decision analytical modelling will be undertaken to
synthesise data from the trial and the literature in order
to determine whether potential benefits of intensive
blood pressure lowering (by lowering the risk of stroke)
are outweighed by potential adverse effects on quality of
life. Ultimately the model analyses will inform whether a
further trial, powered to detect differences in clinical
end-points, is required.
A Markov model will be constructed to consider
intensive target and standard target strategies for blood
pressure lowering in patients with a history of stroke or
TIA. The clinical events of importance in the model are
further stroke events, myocardial infarction (MI) and
other cardiovascular related mortality. Data from the
trial and literature will inform the probability of these
events occurring and the risk reduction afforded by the
alternative strategies. Attached to each health state will
be associated health state utility values (quality of life)
in order that quality-adjusted life years (QALYs) can be
calculated. Quality of life on each treatment strategy will
be obtained from the trial data on EQ-5 D, and previous
studies will inform post-stroke and post-MI values. In
addition, in order that cost-effectiveness analyses can be
More intriguing information
1. The name is absent2. 101 Proposals to reform the Stability and Growth Pact. Why so many? A Survey
3. AGRICULTURAL TRADE IN THE URUGUAY ROUND: INTO FINAL BATTLE
4. THE MEXICAN HOG INDUSTRY: MOVING BEYOND 2003
5. THE UNCERTAIN FUTURE OF THE MEXICAN MARKET FOR U.S. COTTON: IMPACT OF THE ELIMINATION OF TEXTILE AND CLOTHING QUOTAS
6. Are Japanese bureaucrats politically stronger than farmers?: The political economy of Japan's rice set-aside program
7. The name is absent
8. Aktive Klienten - Aktive Politik? (Wie) Läßt sich dauerhafte Unabhängigkeit von Sozialhilfe erreichen? Ein Literaturbericht
9. The name is absent
10. A Bayesian approach to analyze regional elasticities