In the variety of C-termini functions is observed and chaperon-like activity (Sarkar
et al., 2001). Within the cell exist proteins called "molecular chaperones” i.e.
helpers for proper protein folding. They are needed because polypeptides in the
cell could fold in multiple ways, some of them biologically useless (aggregation),
thus there is always a kinetic competition between the correct folding and the
aggregation (Zettlmeissl et al., 1979). The yield of the folded protein will depend
upon the relative rate of two processes. Therefore, for successful folding of a
protein, chaperones minimize the rate of aggregation.
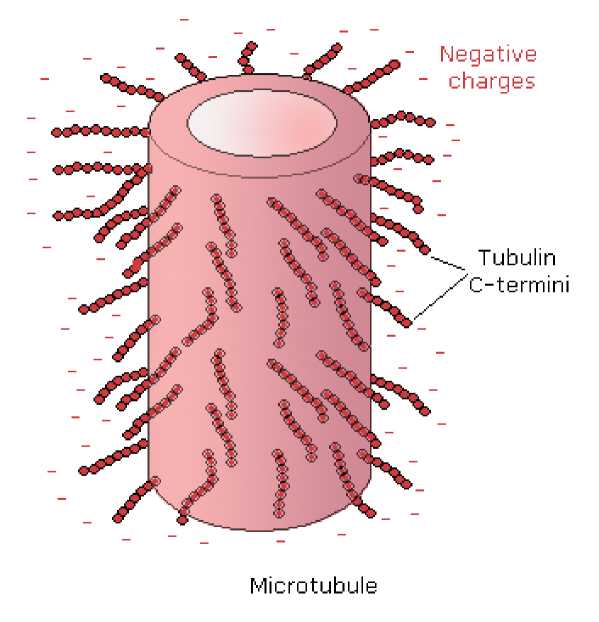
Fig. 21 Tubulin C-termini project from the tubulin subunits and interact with the
microtubule-associated structural and motor proteins, cytoplasmic ions (Ca ++)
and other charged regulator molecules (colchicine, vinblastine). Additional role
for the tubulin tails is chaperon-like activity.
70
More intriguing information
1. The name is absent2. Shifting Identities and Blurring Boundaries: The Emergence of Third Space Professionals in UK Higher Education
3. The Role of Trait Emotional Intelligence (El) in the Workplace.
4. The Impact of EU Accession in Romania: An Analysis of Regional Development Policy Effects by a Multiregional I-O Model
5. Neighborhood Effects, Public Housing and Unemployment in France
6. Educational Inequalities Among School Leavers in Ireland 1979-1994
7. MANAGEMENT PRACTICES ON VIRGINIA DAIRY FARMS
8. The name is absent
9. The name is absent
10. The name is absent