1985), as attachment points for microtubule-associated proteins and cytoplasmic
dynein (Serrano et al, 1984), in the enhancement of the pH sensitivity of the
vinblastine-induced polymerization process, and as possible binding sites for
calcium ions (Solomon, 1977).
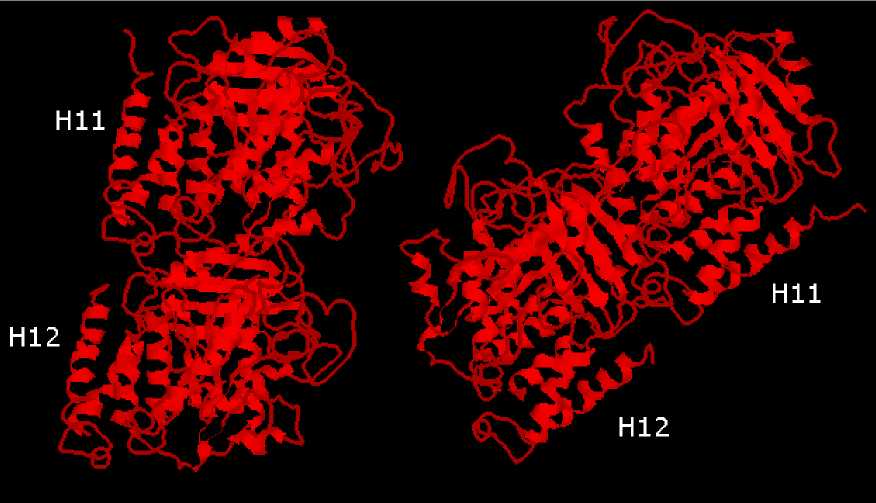
Fig. 20 Tubuiin α∕β-dimer structure derived from electron diffraction study
Nogales et al, 199 ). Tubuln α and β C-termini are shown as helces H11 and
H12, respectively. ITUBpdb file from The Protein Data Bank is used.
The amino acid sequences encoded by β-tubulin genes have revealed a high
level of overall similarity, but significant divergence between their C-termini (Hall
et al., 1985). The pattern of expression of the β-tubulin genes has been studied
in several different human cell lines and has revealed varying levels of and
differential expression in different cell lines. It appears that distinct human
β-tubulin isotypes are encoded by genes whose exon size and number has been
conserved evolutionarily, but whose pattern of expression may be regulated
either coordinately or uniquely (Hall et al., 1985).
69
More intriguing information
1. Incorporating global skills within UK higher education of engineers2. Large Scale Studies in den deutschen Sozialwissenschaften:Stand und Perspektiven. Bericht über einen Workshop der Deutschen Forschungsgemeinschaft
3. The name is absent
4. Short- and long-term experience in pulmonary vein segmental ostial ablation for paroxysmal atrial fibrillation*
5. Are Japanese bureaucrats politically stronger than farmers?: The political economy of Japan's rice set-aside program
6. Enterpreneurship and problems of specialists training in Ukraine
7. A Computational Model of Children's Semantic Memory
8. THE AUTONOMOUS SYSTEMS LABORATORY
9. The name is absent
10. The name is absent