Roberto De Ponti, “Cryothermal Energy Ablation Of Cardiac Arrhythmias 2005: 13
State Of The Art”
the treatment of some forms of ventricular tachycardia5,6, is safe and effective. During surgery,
cryomapping allowed precise localization of the arrhythmogenic substrate by monitoring the
effect of a cryothermal energy application with higher temperature (0 to -15°C) for a limited
time (15-30s), before producing a permanent lesion at -65°C only in the most appropriate site7.
In the 90’s, significant engineering advances allowed the development of systems for
percutaneous cryoablation, consisting of a steerable catheter, apparently not very different from
standard ablation catheters for radiofrequency energy delivery, and a dedicated console (Figure
1). Fluid nitrous oxide is delivered under pressure to the catheter tip through a hollow injection
tube, which runs internally for the whole length of the catheter. In a small chamber inside the tip
electrode, nitrous oxide is made expand and a liquid to gas phase change takes place with heat
extraction from the electrode-to tissue interface. The gas is constantly removed through a second
coaxial lumen inside the catheter, under vacuum. The tip temperature is constantly monitored by
the console, which in turn adjust the nitrous oxide flow to obtain and maintain the preset
temperature. Two systems for cryoablation are currently available. The first is provided by
Cryocath Technologies Inc. (Montreal, Canada) and utilizes 7 or 9 F steerable catheters with 4, 6
or 8 mm long tip electrode. The ablation catheter is connected to a dedicated console, which has
two algorithms available: 1) for cryomapping with slow decrease of the temperature to - 30°C up
for 80 s; 2) for cryoablation with faster decrease of the temperature to -75°C for up to 480 s. In
any case, the target temperature can be manually preset on the console at any value between -30
and -75°C. The second system (CryoCor Inc., San Diego, California, USA) has 10 F steerable
catheters with 6.5 or 10 mm long tip electrodes. The console has a built-in closed loop pre-cooler
for the fluid nitrous oxide, whose flow at the catheter tip is adjusted during the application to
maintain a temperature of -80°C.
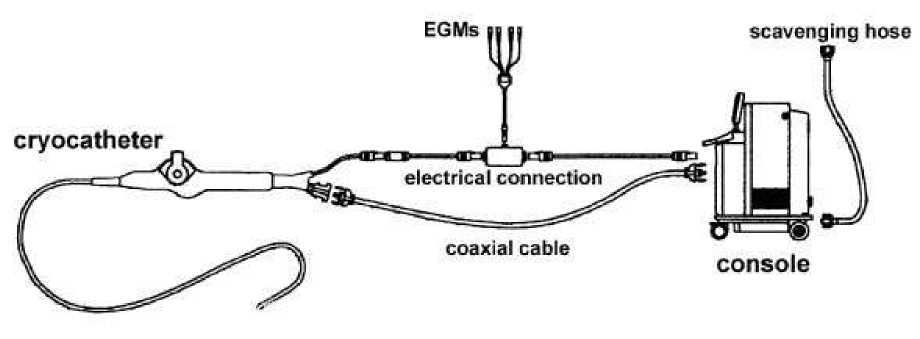
Figure 1. Scheme of cryoablation system. The steerable catheter and the console are
connected by: 1) a coaxial cable, used both to deliver fluid nitrous oxide to the
catheter and to remove separately the gas from the catheter; 2) electrical cable, which
is connected both to the conventional recording system for electrograms (EGMs)
analysis and storage and to the console for reading of the tip temperature. A tank of
fluid nitrous oxide is located inside the console; the gas removed from the catheter to
the console is evacuated through a scavenging hose into the vacuum line of the
electrophysiology laboratory. The system has several sensors to avoid inadvertent
leaks of nitrous oxide into the patient body and to check connections of the different
cables to the console.
Lesion formation by cryothermal energy
Since cryothermal energy has been widely used in surgery, the types of cellular lesion
caused by tissue freezing are well known8. The mechanisms underlying lesion formation by
Indian Pacing and Electrophysiology Journal (ISSN 0972-6292), 5(1): 12-24 (2005)
More intriguing information
1. sycnoιogιcaι spaces2. Regional specialisation in a transition country - Hungary
3. Spectral calibration of exponential Lévy Models [1]
4. The name is absent
5. ‘Goodwill is not enough’
6. The name is absent
7. The name is absent
8. Opciones de política económica en el Perú 2011-2015
9. Has Competition in the Japanese Banking Sector Improved?
10. Improving behaviour classification consistency: a technique from biological taxonomy