Purerfellner H., Aichinger J., Martinek M., Nesser H.J., Janssen J, 14
“Short- and long-term experience in pulmonary vein segmental ostial ablation for
paroxysmal atrial fibrillation”
during follow up, clinical success is primarily based on symptomatic episodes of PAF. It
remains unclear who often and to what extent silent episodes of PAF may have changed the
clinical results of PVI in our patient subgroups.
Future outlook
According to many other working groups worldwide we have changed our ablation
strategy recently: Using a 3 dimensional mapping system (CARTO, Biosense Webster Inc.) an
electroanatomic map of the LA including the mitral valve annulus and the PVs is reconstructed
pre ablation. Thereafter, periostial circumferential atrial lesions are deployed around the septal
and lateral PVs in order to modify the substrate of PAF (Figure 5). In addition, an ablation line
in between the LIPV and the mitral valve is drawn (socalled „mitral isthmus“), which is at times
accompanied by a roof line in the LA connecting the superior PVs. It seems critical to attain
complete lesion sets designed to prevent the spread of focal discharges from withhin the PV to
the rest of the LA (Pappone approach12). At this point in time we are still using a multipolar
circular catheter to be located at the ostial level to monitor and guide PV isolation at the LA-PV
junction. Recently published reports demonstrate higher success rates using circumferential PV
atrial ablation in contrast to segmental ostial PVI both in PAF13 and (even more) in persistent to
permanent forms of AF. An additional advantage of this technique is based on the diminished
occurence of PV stenoses by applying energy >1cm away from the ostial level. However,
additional point lesions at the ostial level of a PV are often required to accomplish complete LA-
PV block.
a
b
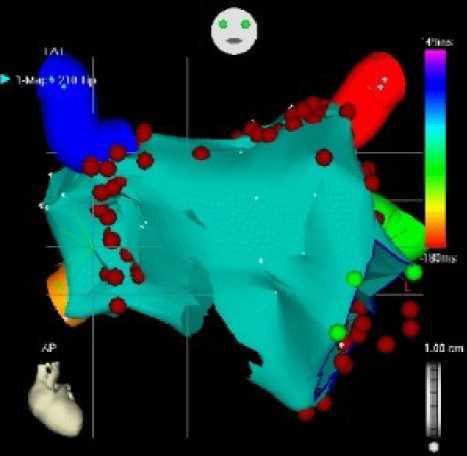
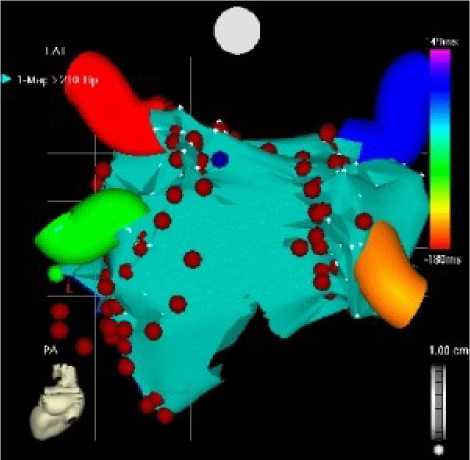
Figure 5: Elecroanatomical Mapping (CARTO): the red dots are representing ablation spots
a) Anteroposterior projection, b) posteroranterior projection
In the meantime a multislice CT imaging modality is available in our institution which
enables the 3 dimensional representation of a patient´s individual cardiac anatomy (Figure 6). In
addition, worldwide intensive collaboration within different manufacturers of cardiac
visualization systems is ongoing to enable "3 D image integration" which will allow a direct
transfer of imaging files to the mapping system at work. By this, a direct transfer of the
individual electrical activation to the individual patient´s anatomy seems feasible ("image
guiding“).
Indian Pacing and Electrophysiology Journal (ISSN 0972-6292), 6(1): 6-16 (2006)
More intriguing information
1. The Formation of Wenzhou Footwear Clusters: How Were the Entry Barriers Overcome?2. LABOR POLICY AND THE OVER-ALL ECONOMY
3. Death as a Fateful Moment? The Reflexive Individual and Scottish Funeral Practices
4. FDI Implications of Recent European Court of Justice Decision on Corporation Tax Matters
5. Dynamiques des Entreprises Agroalimentaires (EAA) du Languedoc-Roussillon : évolutions 1998-2003. Programme de recherche PSDR 2001-2006 financé par l'Inra et la Région Languedoc-Roussillon
6. A Regional Core, Adjacent, Periphery Model for National Economic Geography Analysis
7. The name is absent
8. The name is absent
9. Determinants of Household Health Expenditure: Case of Urban Orissa
10. Using Surveys Effectively: What are Impact Surveys?