J.M. DiPirro, M.B. Kristal / Brain Research 1014 (2004) 22-33
27
binding affinity for the A-opioid receptor that is 100 times
greater than its affinity for y and n receptors [24,28,36].
DAMGO was dissolved in 0.9% physiological saline and
injected within 1 h of entering solution.
4.2. Results and discussion
Of the 101 rats tested in Experiment 2, three rats failed to
eat placenta or beef control during testing, and two rats had
inaccurate cannula placements. Only the data from the
remaining 96 rats were used for analysis.
4.2.1. POEF and l-receptor-mediated antinociception
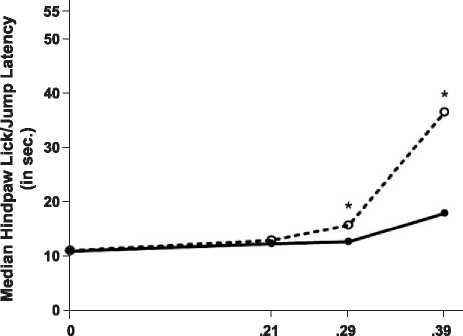
The effect of POEF on l-mediated antinociception is
depicted in Fig. 2. POEF, ingested as placenta, significantly
attenuated the antinociception induced by DAMGO at both
the high (0.39 nmol) dose (v2[1, n =24] = 4.0, p < 0.05), and
moderate (0.29 nmol) dose (v2[1, n =24] = 4.2, p < 0.05).
Placenta ingestion did not affect response latency in rats
injected with the low (0.21 nmol) dose of DAMGO (v2[1,
n =24]=0, p>0.05), or with vehicle (v2[1, n=24] =0,
p>0.05). These data indicate that the A-opioid receptor is
not involved in the antinociception-enhancing effect of
POEF, and instead may be involved in an antinociception-
inhibiting effect observed after treatment with large amounts
of placenta or amniotic fluid.
The finding that POEF does not potentiate the A anti-
nociception produced by DAMGO was somewhat surpris-
ing in light of previous evidence showing that POEF
induces a marked potentiation of antinociception produced
by i.c.v. morphine [19], an opiate agonist that acts predom-
inantly at A receptors [25,52,84]. Several explanations for
this apparent discrepancy are possible. The simplest and
Dose of DAMGO (nmol, I.C.V.)
Fig. 2. Attenuation by placenta ingestion of A-opioid receptor-mediated
antinociception. Female rats were fed 1.0 g placenta (.) or control
substance (o) 10 min before they were injected with DAMGO (0, 0.21,
0.29, or 0.39 nmol, i.c.v.). Pain threshold is represented by median response
latency (in seconds) on a 52 jC hotplate test 30 min after DAMGO
injection (n = 11-13 rats/group). *Significantly different from control-fed
treatment group at the corresponding DAMGO dose ( p < 0.05, median test).
most likely is that the enhancing mechanism of POEF does
not involve the A receptor. Morphine is considered to be a
non-selective A-preferring agonist, with binding affinity at
each of the three types of opioid receptor [25]. Although
antinociception generated by this drug is attributed primarily
to the activation of A receptors [52,84], it can also result
from stimulation of either y [36,94] or n receptors [86].
These findings, together with data from Experiment 2,
suggest that the dramatic potentiation of non-receptor-spe-
cific morphine antinociception by POEF represents the
modulation of opioid activity at the y receptor, n receptor,
or both. Whether POEF ingestion affects morphine activity
simultaneously at all three opioid receptors is unknown, but
if it does, then enhancement occurs because the elevation of
y or n activity is robust enough to outweigh a coincident and
functionally opposite inhibition of A activity. The results of
Experiments 1 and 2 are clearly consistent with such a
proposition. Therefore, the differential effect of POEF on
antinociception generated by DAMGO and morphine can
likely be attributed to the fact that the A receptor is not a
substrate for the enhancement effect.
Alternatively, the effect of ingested POEF on antinoci-
ception induced by selective (DAMGO) and non-selective
(morphine) A receptor agonism may reflect an enhancing
mechanism that depends on combined activation of two or
more opioid receptors [3,57,83]. If such an interaction were
necessary for an enhancement of A antinociception by POEF
ingestion, then antinociception produced by morphine or
other nonspecific opioid receptor agonists should be en-
hanced, but not that produced by the A-receptor-specific
agonist DAMGO. However, the results of Experiment 2
show that selectively induced A activity is actually attenu-
ated by POEF ingestion. Therefore, again, it seems unlikely
that the A receptor is involved in the POEF enhancement
effect.
Last, it is possible that the different effects of POEF
ingestion on DAMGO- and morphine-induced antinoci-
ception reflect differences between the agonists them-
selves rather than reflect the effect of POEF on A-
mediated phenomena [64]. If morphine and DAMGO
activate distinct A-receptor-containing antinociception
pathways, as appears to be true of morphine and h-
endorphin [59,91], then it is conceivable that an enhanc-
ing action of POEF on A-opioid activity might have been
obscured in the present study by the selection of DAMGO
as the A agonist.
This experiment represents the first systematic documen-
tation of the antinociception-attenuating ability of POEF
ingestion. It is not, however, the first evidence that POEF
can exert a negative modulatory effect on opioid-mediated
processes. Amniotic fluid ingestion attenuates contralateral
circling induced by unilateral injection of morphine into the
VTA [90]. Because increases in forward locomotion after
intra-VTA opioid injection are thought to be mediated by A
or y receptor activation of dopaminergic projections from
the VTA to nucleus accumbens [10,34,47] and because
More intriguing information
1. IMMIGRATION POLICY AND THE AGRICULTURAL LABOR MARKET: THE EFFECT ON JOB DURATION2. The name is absent
3. Legal Minimum Wages and the Wages of Formal and Informal Sector Workers in Costa Rica
4. The name is absent
5. Empirical Calibration of a Least-Cost Conservation Reserve Program
6. The name is absent
7. The name is absent
8. Investment in Next Generation Networks and the Role of Regulation: A Real Option Approach
9. Consciousness, cognition, and the hierarchy of context: extending the global neuronal workspace model
10. Research Design, as Independent of Methods