26
J.M. DiPirro, M.B. Kristal / Brain Research 1014 (2004) 22-33
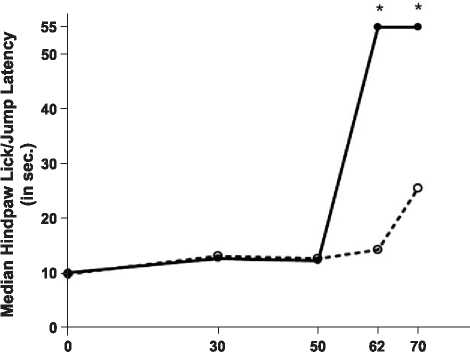
Dose of DPDPE (nmol, I.C.V.)
Fig. 1. Enhancement by placenta ingestion of y-opioid receptor-mediated
antinociception. Female rats were fed 1.0 g placenta (.) or control
substance (o) 10 min before they were injected with DPDPE (0, 30, 50, 62,
or 70 nmol, i.c.v.). Pain threshold is represented by median response latency
(in seconds) on a 52 °C hotplate test 10 min after DPDPE injection (n = 5 -
8 rats/group). *Significantly different from control-fed treatment group at
the corresponding DPDPE dose ( p < 0.05, median test).
two lowest doses (30 and 50 nmol) of DPDPE ( p>0.05,
median test).
The results of Experiment 1 clearly demonstrate that
POEF ingestion (as placenta) enhances y-mediated antino-
ciception in the hotplate test in a dose-dependent fashion.
Because this assay measures purposeful antinociceptive
responses that are coordinated at a level of the neuraxis
above the spinal cord [65], these results indicate that
ingested POEF modulates supraspinally organized antinoci-
ception. Given the route of DPDPE injection (i.c.v.) and the
post-injection interval of antinociception determination (10
min), the DPDPE effect in the present study is likely due to
an action at supraspinal receptors. This points to the
conclusion that POEF enhances antinociception at supra-
spinal y1 sites [76]. However, the present study did not test
whether POEF can also affect y2 activity; in the rat, at some
brain sites, agonists selective for the y2 receptor are more
effective than are y1 agonists in the production of antinoci-
ception [62,75].
3.2.2. POEF and d-receptor-mediated circling
The effect of DPDPE on contralateral circling is depicted
in Table 1. At all doses, DPDPE injection alone produced no
apparent motor effects in rats at the time of the hotplate test,
suggesting that doses used in the present study were
relatively low. In contrast, when combined with placenta
ingestion, DPDPE injection induced circling in a significant
proportion of rats (86%), but only at the highest dose of
DPDPE (p < 0.05, Fisher exact probability test).
These results indicate that POEF ingestion can modulate
motor components of y-opioid activity as well as the
antinociceptive component. This is consistent with a previ-
ous report that placenta ingestion (1.0 g) roughly doubles
the potency of DPDPE in the induction of circling [18]. It is
important that the results of Experiment 1 show that at
different doses of DPDPE, the motor-enhancing effects of
POEF can be dissociated from the antinociception-enhanc-
ing effects. At the highest dose of DPDPE (70 nmol),
placenta ingestion enhanced both antinociception and cir-
cling in the same rats. In contrast, at 62 nmol DPDPE,
placenta ingestion enhanced antinociception, but had no
effect on circling. The finding that POEF induced an
elevation in paw-lick/jump latency—both with and without
the simultaneous induction of circling, depending on dose of
DPDPE—suggests that the elevated response latencies
manifested in the hotplate test represent a potentiation of
opioid antinociception, and not a confounding motor effect.
In addition, because the mechanism underlying locomotor
activation induced by i.c.v. y agonists is thought to involve
enhanced dopamine release in the nigrostriatal pathway
[14], these data suggest that POEF ingestion might modu-
late opioid activity in that system.
4. Experiment 2: M-opioid receptors
The effect of placenta ingestion on the antinociception
produced by the i.c.v. injection of each of four doses of the
A-opioid-preferential agonist DAMGO was measured.
4.1. Method
4.1.1. Subjects
One hundred one virgin rats, demonstrating normal
estrous cyclicity, were used.
4.1.2. Design
The design was a 2 × 4 between-subjects factorial: En-
hancer (1.0 g placenta or 1.0 g control substance) × Dose of
A agonist (0.00, 0.21, 0.29, or 0.39 nmol DAMGO in 4.0 Al,
i.c.v.). Rats were randomly assigned to one of eight exper-
imental conditions and tested only once.
4.1.3. Drug
The A-receptor-selective agonist DAMGO (a gift from
NIDA) was used. DAMGO was chosen because it displays a
Table 1
Proportion of group showing stereotypic circling at the time of
antinociception measurement (Experiment 1)
|
DPDPE dose |
Enhancer | |
|
Placenta |
Control | |
|
0 |
0/7 |
0/8 |
|
30 |
0/8 |
0/8 |
|
50 |
0/8 |
0/7 |
|
62 |
0/5 |
0/6 |
|
70 |
6/7a |
2/6 |
a Significantly different from control-fed treatment group at the
corresponding DPDPE dose ( p < 0.05, Fisher exact probability test).
More intriguing information
1. The Value of Cultural Heritage Sites in Armenia: Evidence From a Travel Cost Method Study2. Investment in Next Generation Networks and the Role of Regulation: A Real Option Approach
3. The WTO and the Cartagena Protocol: International Policy Coordination or Conflict?
4. Are Public Investment Efficient in Creating Capital Stocks in Developing Countries?
5. Midwest prospects and the new economy
6. Dual Track Reforms: With and Without Losers
7. EMU's Decentralized System of Fiscal Policy
8. Regional Intergration and Migration: An Economic Geography Model with Hetergenous Labour Force
9. A Rational Analysis of Alternating Search and Reflection Strategies in Problem Solving
10. The Prohibition of the Proposed Springer-ProSiebenSat.1-Merger: How much Economics in German Merger Control?