852
DI PIRRO, THOMPSON AND KRISTAL
were terminated at 8 s to avoid tissue damage. During testing,
rats were restrained in 5×21-cm opaque, polyvinyl tubes to
which they had been habituated for 1 h∕day for 5 days prior to
testing.
Orogastric infusions of amniotic fluid (AF) and control fluid
were performed through PE 160 tubing, attached to a blunted
18-ga needle fitted to a 0.25-ml, glass, tuberculin syringe. Each
rat was habituated to the intubation procedure (with a blank in-
fusion) once each day for 5 days prior to testing.
Procedure
The overall design of the experiment was a 2 × 2 × 2 facto-
rial: Antagonist [QN (8 mg∕kg, SC); saline vehicle, Veh] ×
Agonist [morphine sulfate, MS (3 mg∕kg, IP); saline, Sal] ×
Enhancer [amniotic fluid, AF (0.25 ml, PO); control fluid, BB].
The dose of QN chosen was based on reported differences in
potency between naltrexone and QN, and on the reported thresh-
old for selective peripheral action (1, 11, 12). Rats were tested
at both levels of the Agonist factor in a balanced design. Tests
were separated by a 1-week interval.
At the start of the test, each rat was injected with the antago-
nist or vehicle. Twenty minutes later, a baseline TFL was deter-
mined for each rat, followed immediately by an injection of
agonist or vehicle. The dose of morphine sulfate was chosen be-
cause it usually produces a small (about 15% increase from
baseline TFL) but reliable amount of analgesia; this level of an-
algesia enables us to detect enhancement without the production
of too many responses at the 8-s ceiling. Enhancer or control
fluid was infused 15 min after the injection of agonist. Fifteen
minutes after infusion, a second TFL was determined. The mean
percent change from baseline TFL was calculated for each rat,
and served as the dependent variable for all the experiments re-
ported here.
Amniotic fluid for orogastric intubation was collected from
donor rats euthanized with CO2 on Day 21 of pregnancy, and
stored in a manner previously described (4,6). The control sub-
stance for AF, beef bouillon (BB), was prepared from Wyler’s
Instant Beef Bouillon diluted to half the strength indicated in the
manufacturer’s instructions, and stored and administered in a
manner identical to that of the AF.
RESULTS AND DISCUSSION
The results of Experiment 1 are depicted in Fig. 1.
Baseline TFLs did not differ between Antagonist groups (QN:
mean = 3.80±0.04 s; Veh: mean = 3.67±0.05 s), F(l,28) =
3.27, p>0.05. Because the baseline TFLs were measured after
the Antagonist treatment, this result indicates that treatment with
QN did not affect pain threshold before the Agonist and En-
hancer were administered.
The 3-way ANOVA (Antagonist × Agonist × Enhancer)
revealed a significant main effect of Antagonist, F(l,28) = 6.07,
p<0.025; this was attributable to the fact that MS-treated rats
injected with QN showed lower pain thresholds than did those
injected with the antagonist vehicle.
There was also a significant Agonist × Enhancer interaction,
F(l,28) = 6.25, p<0.02. The subsequent simple-effects probe of
this interaction indicated that AF infusion enhanced the analge-
sia produced by morphine; MS + AF treatment resulted in
higher pain thresholds than did MS + BB treatment, F( 1,56) =
9.2, p<0.01, or than Sal + AF treatment, F(l,28) = 40.39,
p<0.001.
Although the results of the 3-way ANOVA indicate that en-
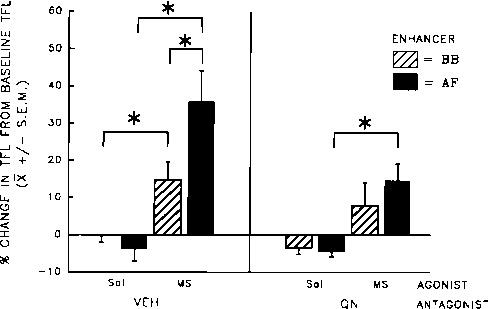
FIG. 1. Mean percent change (±S.E.M.) from baseline tail-flick latency
(TFL) of rats pretreated with systemic antagonist [quaternary naltrexone
(QN) or vehicle (VEH)], then treated with systemic agonist [morphine
(MS) or saline (Sal)] and an orogastric infusion of enhancer [amniotic
fluid (AF) or beef bouillon control (BB)]. n = 8∕group; *p<0.01.
hancement of analgesia by AF does not require the involvement
of peripheral opioid receptors, the analysis did not provide a di-
rect test of the effect of AF on analgesia under the QN condi-
tion. Therefore, we used subsequent pairwise comparisons to test
the effect of various combinations of Agonist and Enhancer
among only QN-injected rats (i.e., AF + MS vs. BB + MS,
AF + MS vs. AF + Sal, and BB + MS vs. BB + Sal). These
comparisons confirmed that AF enhanced the analgesia produced
by MS in QN-treated rats; the MS + AF Group showed analge-
sia whereas the MS + BB Group did not [MS + AF vs. Sal
+ AF: F(l,7)= 17.36, p<0.01; MS + BB vs. Sal + BB:
F(l,7) = 2.50, p>0.05].
These results indicate that although systemic QN attenuated
morphine-induced analgesia, by elimination of the peripheral ef-
fects of morphine, AF ingestion still enhanced the remaining an-
algesia (that attributable to the central effects of morphine).
Therefore, ingested POEF affects at least the central site of ac-
tion of morphine on analgesia.
Experiment 2
In the second experiment, we tested whether amniotic fluid
ingestion enhances analgesia induced by a central injection of
morphine. Experiment 2A examined this possibility when pe-
ripheral opioid receptors were active; Experiment 2B examined
the possibility with peripheral opioid receptors blocked by sys-
temic administration of QN.
Experiment 2A: Central Morphine Administration and Active
Peripheral Receptors
METHOD
Subjects
The subjects were 28 nulliparous, female, Long-Evans (hood-
ed) rats, 90-150 days of age, weighing 290±5.1 g. All rats
were morphine-naive, but had participated in a study 4-6 weeks
prior to this experiment in which they received a single TFL test.
Initially, the rats were maintained identically to the rats in Ex-
periment 1. After surgery for implantation of indwelling intrace-
rebroventricular (ICV) cannulae, they were housed individually
More intriguing information
1. The name is absent2. Monetary Policy News and Exchange Rate Responses: Do Only Surprises Matter?
3. Climate change, mitigation and adaptation: the case of the Murray–Darling Basin in Australia
4. GENE EXPRESSION AND ITS DISCONTENTS Developmental disorders as dysfunctions of epigenetic cognition
5. Globalization, Divergence and Stagnation
6. Distortions in a multi-level co-financing system: the case of the agri-environmental programme of Saxony-Anhalt
7. The name is absent
8. The name is absent
9. The name is absent
10. The name is absent