POEF AND QUATERNARY NALTREXONE
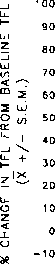
φ = A F / M S, n = 5
0—0 = BB∕MS, n = 6
∙--∙ = AF∕SAL, n = 6
O--O = BB∕SAL, n = 7
TIME IN MIN AFTER FLUID INFUSION
853
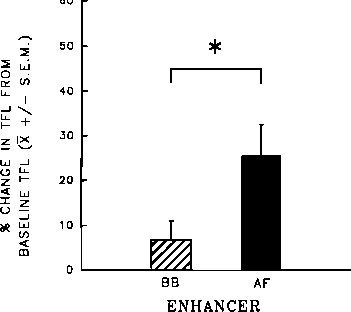
FIG. 3. Mean percent change (±S.E.M.) from baseline tail-flick latency
(TFL), at 20 min after orogastric infusion, of rats pretreated with sys-
temic antagonist [quaternary naltrexone (QN)], then treated with central
agonist [morphine (MS)] and an orogastric infusion of enhancer [amni-
otic fluid (AF) or beef bouillon control (BB)]. n = 7∕group; *p<0.05.
FIG. 2. Mean percent change (±S.E.M.) from baseline tail-flick latency
(TFL) of rats treated with central agonist [morphine (MS) or saline
(SAL)] and an orogastric infusion of enhancer [amniotic fluid (AF) or
beef bouillon control (BB)]. *p<0.0001.
in 32×20×20-cm, free-standing, clear plastic cages. Food and
water were available ad lib, except for a 3-h period prior to,
and during, testing.
Apparatus
Pain-threshold testing and orogastric intubation were per-
formed the same as in Experiment 1.
Drug microinjections were performed using a Harvard micro-
infusion pump (Model 944).
Procedure
The overall design of the experiment was 2 × 2 × 2 factorial:
Agonist [morphine, MS (2.5 μg in 2 μl, ICV); saline, Sal] ×
Enhancer [AF, BB (0.25 ml, PO)] × Time (20 and 40 min af-
ter orogastric infusion). Each rat was randomly assigned to one
Agonist × Enhancer group and tested at both postinfusion time
intervals.
Rats were anesthetized during surgery with 40 mg∕kg, IP,
pentobarbital sodium (65 mg∕ml) after premedication with 4 mg∕
kg, SC, atropine sulfate (5 mg∕ml) to suppress mucus secretion.
Each rat was treated with an antibiotic (Combiotic, 0.5 cc, IM)
as a prophylactic measure. The intracerebroventricular implant
consisted of a 22-ga stainless-steel guide cannula (Plastic Prod-
ucts) inserted into the right lateral ventricle according to the fol-
lowing coordinates: A-P=—0.2 mm (bregma); L=-2.4 mm
(center of midsagittal sinus); D-V =-3.1 mm (cortex), with the
incisor bar positioned 5 mm above the interaural line (10). The
guide cannula was anchored to the skull with dental cement af-
fixed to 0-80 stainless steel screws. During microinjection, the
internal cannula protruded 1 mm beyond the tip of the guide
cannula. The drug was delivered intraventricularly at a rate of 1
μl∕min.
The dose of morphine used (2.5 μg) was chosen so as to
produce a near-threshold response (10-20% average increase in
TFL in pilot rats). This level of elevation is behaviorally equiv-
alent to the amount of analgesia produced by the dose of mor-
phine administered during systemic injection (3 mg∕kg) in
Experiment 1.
Rats were allowed a 2-week recovery period before behav-
ioral testing began. They were habituated to the removal of can-
nula caps daily for at least 5 days prior to the first day of testing.
At the start of the test, baseline pain threshold was deter-
mined for each rat, followed immediately by an injection of
morphine or vehicle into the right lateral ventricle. Ten minutes
after the end of the injection, AF or BB was infused orogastri-
cally. Pain threshold was again measured 20 and 40 min after
the infusion. The dependent measures were calculated as percent
change in pain threshold from pretreatment baseline at 20 and
40 min postinfusion.
At the conclusion of the study, each rat was overdosed with
pentobarbital sodium, IP, and given a 1.0-μl ICV injection of
methyl blue dye to determine subsequently whether the cannula
was located in the ventricle. The brains were perfused and re-
moved, and the placement verified by histological examination
of frozen sections (40 μ) stained with cresyl violet. Data for
statistical analysis were taken only from rats whose placements
fell within the right lateral ventricle. Four rats were excluded
from the analysis on the basis of cannula placement.
RESULTS AND DISCUSSION
The results from Experiment 2A are depicted in Fig. 2.
Baseline TFLs did not differ significantly among the groups.
They ranged from 3.64±0.09 s in the AF + Sal Group to
3.48 ±0.17 s in the BB + MS Group, F(3,20)<1.0.
A 3-way ANOVA (Agonist × Enhancer × Time) revealed
a significant Agonist × Enhancer interaction, F(l,20) = 29.09,
^<0.0001. The probe of the interaction for simple effects indi-
cated that, as planned, our dose of centrally administered mor-
phine in the absence of amniotic fluid ingestion produced a small
(10%) increase in TFL. This increase was not statistically sig-
nificant, F(l,20)= 1.77, p>0.05. In contrast, centrally adminis-
tered morphine in conjunction with amniotic-fluid ingestion
produced a significant elevation in TFL (analgesia) at both time
measures, F(l,20) = 73.07, p<0.0001; MS + AF treatment
produced about a 92% elevation from baseline at 20 min and
about a 98% elevation from baseline at 40 min, whereas MS +
BB treatment produced only about a 10% elevation in pain
threshold. In rats receiving central saline injections, no change
More intriguing information
1. The name is absent2. CROSS-COMMODITY PERSPECTIVE ON CONTRACTING: EVIDENCE FROM MISSISSIPPI
3. The name is absent
4. Staying on the Dole
5. Studies on association of arbuscular mycorrhizal fungi with gluconacetobacter diazotrophicus and its effect on improvement of sorghum bicolor (L.)
6. The name is absent
7. Transport system as an element of sustainable economic growth in the tourist region
8. Une Classe de Concepts
9. Conditions for learning: partnerships for engaging secondary pupils with contemporary art.
10. The name is absent