KINETICS ON THE MICROBIAL SCALE
49
|
Z = "ACTIVATED" |
COUPLING ENZYME | |
|
( I ) |
R∣t∙∣ + z = |
(ZR)iti |
|
(2) |
Oi +(ZR)itl = |
∣Z0∣it∣ + Ri |
|
(3) |
∣zo∣itl+ p = |
IZPi + oitl |
(4) ∣ZP} * ADP = Z t ATP + H2O
FIG. 4- A POSSIBLE MECHANISM FOR PHOSPHATE
FIXATION IN THE ELECTRON TRANSPORT
PATHWAY
algebra of the kinetic relations. Clearly we are going to end up with a
large number of parameters “to be determined" in the expressions we
will derive, simply because of the large number of reactions and elemen-
tary steps. The usual objective of “fitting the data to the theory” and
evaluating the constants is obviously out of the question. The best we
hope for is a set of equations which we can subject to certain tests.
These will necessarily be of the plausibility type, i. e., can we find a set
of physically meaningful parameters such that the mathematical system
is a reasonable model of the living system.
In Figure 5 I outline the procedure for obtaining the rate equation for
ALL SYMBOLS NOW REPRESENT CONCENTRATIONS
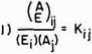
(THE K,∙j ARE EQUILIBRIUM CONSTANTS)
<f∣>i ∙^{'⅛0lΦij- b⅛(o*⅛)
THE fij ARE FORWARD
rate constantsithe
b*j ARE REVERSE RATE
CONSTANTS
*(J0b∣i.∣l ∙ E°. (CONSTANT 1
,Rl,∣ ANOTHE CONSTANTS kij , 11∣ , Ц,, E°
(2) (rt)i .∑(fij(θJ)ijiRitl - bijRi (Jθ)ijit∣)
(⅞)j .∑uj(Jo)ijitl - bij(J)ij oitl I
AT STEADY STATE: (rι)i∙(⅞)∣∙ ( Γj )∣
CONSERVATION OF ENZYME i :(Ej) ♦ ),j ♦ •••
(3) r . f* (OiXRld) - b* (OjdXRi)
WHERE f*,b*∣ ARE FUNCTIONS OF (Aj).(O∣). R∣ .O1
FIG.5-RATE EQUATIONS ANO ALGEBRA FOR TYPICAL STEP IN A PATHWAY
More intriguing information
1. Fiscal Rules, Fiscal Institutions, and Fiscal Performance2. The name is absent
3. The name is absent
4. The name is absent
5. Unemployment in an Interdependent World
6. The name is absent
7. Motivations, Values and Emotions: Three Sides of the same Coin
8. Automatic Dream Sentiment Analysis
9. Clinical Teaching and OSCE in Pediatrics
10. Fortschritte bei der Exportorientierung von Dienstleistungsunternehmen