HIGHLEY ET AL
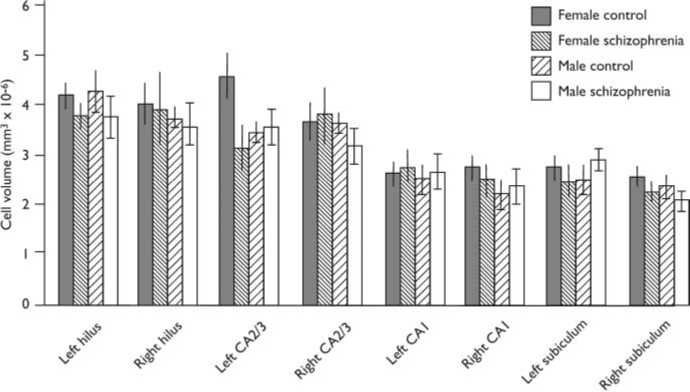
Fig. 2 Pyramidal cell volumes for hippocampal subregions (bars show means and standard error of the
mean). Cases have been subdivided according to gender and disease category.
OCE can be used to estimate the
percentage of observed relative variance,
(s.d.=x)2, of each measure which is
accounted for by true inter-individual
variance, as opposed to the stereological
volume estimate (West & Gundersen,
1990; West, 1999). Ideally, this should be
greater than 50%. This was true for all
measures of VN (all 580.2%). The conclu-
sion to be drawn is that the measures are
of adequate reliability and accuracy.
Effects of diagnosis, gender
and side
A bar chart of mean cell volume is given in
Fig. 2. The mean cell volumes (standard
deviations in parentheses) for the subfields
were as follows:
(a) hilus: 4.0861076 (0.8461076) for
controls and 3.8261076 (1.236
1076) mm3 for patients;
(b) CA2/3: 3.8861076 (1.0961076)mm3
for controls and 3.4561076 (1.056
1076) mm3 for patients;
(c) CA1: 2.5461076 (0.5961076)mm3
for controls and 2.6061076 (0.676
1076) mm3 for patients;
(d) subiculum: 2.5261076 (0.576
1076) mm3 for controls and 2.256
1076 (0.4661076) mm3 for patients.
There was no significant effect for diag-
nosis, gender or side for any subfield. Thus,
for the hilus, all F(1,24)41.22, P50.2021;
for the CA2/3 subfield, all F(1,24)43.25,
P50.0842; for the CA1 subfield, all
F(1,23)41.35, P50.2574; for the subiculum,
all F(1,23)42.19, P50.1522.
DISCUSSION
The main finding in this study is an absence
of size change in hippocampal pyramidal
neurons in schizophrenia. There have been
five earlier studies of this parameter of
which we are aware (Christison et al,
1989; West & Gundersen, 1990; Benes et
al, 1991, 1998; Arnold et al, 1995; Zaidel
et al, 1997; West, 1999); two of them
found no change, and three found a de-
crease in size in schizophrenia. All had
comparable numbers of cases of schizo-
phrenia to the number in the present study.
Control case numbers were similar to our
study in four studies but were larger in
one other negative study. All studies used
the Nissl stain. Only one previous study in
addition to ours looked at both sides of
the brain, and only our study sampled the
hippocampus throughout its full extent.
We addressed the potential of regional
specificity of changes in schizophrenia by
dividing the hippocampus into four sub-
fields. We did not further divide our hippo-
campal subfields into anterior and posterior
halves. It is thus possible that changes in
one half (anterior or posterior) of a subfield
might have been masked or ‘diluted’ by var-
iance in the other half. In a meta-analysis of
hippocampal volumes in schizophrenia
assessed by MRI it was found that inclusion
of the amygdala, abutting on the anterior
hippocampus, in the region of interest sig-
nificantly increased the size of the reduction
in volume seen in schizophrenia. The re-
commendation was made that in future re-
search relative alterations in anterior and
posterior hippocampus in schizophrenia
should be assessed separately (Nelson et
al, 1998). It is also possible that our study
might have failed to detect a ‘true’ reduc-
tion in cell size in some hippocampal sub-
fields because of the small sample size
(type II error).
Decreases in neuronal size have been re-
ported for other regions of the brain in
schizophrenia - the dorsolateral prefrontal
cortex, anterior cingulate cortex, cerebellar
Purkinje cells, substantia nigra and locus
caeruleus - but not in the motor cortex or
calcarine cortex (reviewed by Harrison,
1999). Further studies will be needed before
the primacy of these changes in the disease
can be judged.
ACKNOWLEDGEMENTS
This work was funded by grants from the UK Medi-
cal Research Council and the Wellcome Trust. We
thank Drs S. J. Cooper and B. Herron for assistance
with cliinical assessment and post-mortem brain
removal respectively for some of the cases included
in this study.
REFERENCES
Altshuler, L. L., Casanova, M. F., Goldberg, T. E., et al
(1990) The hippocampus and parahiippocampus in
schizophrenia, suicide, and control braiins. Archives of
General Psychiatry, 47, 1029^1034.
American Psychiatric Association (1994) Diagnostic
and Statistical Manual of Mental Disorders (4th edn)
(DSM ^ IV).Washington, DC: APA.
Arnold, S. E., Franz, B. R., Gur, R. C., etal (1995)
Smalller neuron size in schizophreniia in hippocampal
subf ields that mediate cortical ^ hippocampal
iinteractions. American Journal of Psychiatry, 152,
738^748.
Benes, F. M., Sorensen, I. & Bird, E. D. (19 91)
Reduced neuronal size in posterior hippocampus of
schizophrenic patiients. Schizophrenia Bulletin, 17,
597^608.
__ , Kwok, E.W.,Vincent, S. L., et al (1998) A
reduction of nonpyramidal cells iin sector CA2 of
schizophrenics and maniic depressives. Biological
Psychiatry, 44, 88^97.
Bogerts, B., Meertz, E. & Schonfeldt-Bausch, R.
(1985) Basall gangllia and liimbic system pathology in
schizophrenia. A morphometriic study of braiin vollume
and shrinkage. Archives of General Psychiatry, 42,
784^791.
Bruton, C. J., Crow, T. J., Frith, C. D., et al (1990)
Schizophrenia and the brain: a prospective cliniico-
neuropathological study. Psychological Medicine, 20,
285^304.
Christison, G. W., Casanova, M. F., Weinberger, D. R.,
et al (1989) A quantitative investigation of hiippocampal
pyramidal celll siize, shape, and variiability of orientatiion in
schizophrenia. Archives of General Psychiatry, 46,
11027^1032.
Falkai, P. & Bogerts, B. (1986) Cell loss iin the
hiippocampus of schizophrenics. European Archives of
Psychiatry and Neurological Sciences, 236, 1154^161.
416
More intriguing information
1. A Rare Case Of Fallopian Tube Cancer2. The name is absent
3. The purpose of this paper is to report on the 2008 inaugural Equal Opportunities Conference held at the University of East Anglia, Norwich
4. Design and investigation of scalable multicast recursive protocols for wired and wireless ad hoc networks
5. Wounds and reinscriptions: schools, sexualities and performative subjects
6. How to do things without words: Infants, utterance-activity and distributed cognition.
7. DETERMINANTS OF FOOD AWAY FROM HOME AMONG AFRICAN-AMERICANS
8. Strengthening civil society from the outside? Donor driven consultation and participation processes in Poverty Reduction Strategies (PRSP): the Bolivian case
9. AJAE Appendix: Willingness to Pay Versus Expected Consumption Value in Vickrey Auctions for New Experience Goods
10. The name is absent