serovar Typhimurium requires an alternative s factor, s28
(Frye et al., 2006; Zhao et al., 2007). Recall that the RNA
polymerase s subunit is a dissociable promoter specificity
factor that binds to core RNA polymerase (E) to form the
RNA polymerase holoenzyme (Es), which can recognize
promoter sequences and initiate transcription (Burgess
et al., 1969). Most bacteria contain multiple s factors that
recognize different promoter -10 and -35 elements. A
primary s factor (s70 in E. coli) drives the transcription of
genes with ‘housekeeping’ functions, while a number of
alternative s factors direct transcription of particular sets
of genes in response to environmental signals or
stresses, or function to control development (Ishihama,
2000; Gruber and Gross, 2003). s28, which is encoded by
the fliA gene, is the most widely distributed alternative s
factor (Koo et al., 2009; Smith and Hoover, 2009), and
controls the transcription of operons required for flagellar
filament assembly and for the regulation of motility and
chemotaxis in a large number of Gram-positive and Gram-
negative bacteria (Chilcott and Hughes, 2000).
Most studies on transcription activation by CRP have
been concerned with promoters recognized by RNA
holoenzyme containing s70 (Es70). Here, we report the first
investigation into the direct regulation by CRP of transcrip-
tion by RNA polymerase containing s28 (Es28). We show
that aer is transcribed from a single s28-dependent pro-
moter that is activated by CRP binding at a location dif-
ferent from any previously characterized CRP-activated
promoter. We also show that CRP directly activates tran-
scription from a second s28-dependent promoter that has
a similar organization.
Results and discussion
Transcription from the aer regulatory region requires
both CRP and s28 in vivo
To study the effects of CRP and s28 on expression of aer,
we cloned a DNA fragment covering the aer gene regula-
tory region (aer200; Hollands et al., 2007) into a low-copy-
number lac expression vector, pRW50, and we measured
the activity of the resulting aer200::lacZ fusion in E. coli
K-12 Dlac strain M182 and derivatives containing deletions
of either the crp or fliA gene. Results presented in Fig. 1A
(black lines) show that, in M182, there isa large increase in
promoter activity during late exponential phase that
decreases on entry into stationary phase. This is consistent
with the findings of Barembruch and Hengge (2007), who
observed a similar pattern of expression for the s28-
dependent flgM promoter, and correlates with an accumu-
lation of s28 protein during late exponential phase followed
by a decline in s28 levels once the culture enters stationary
phase (K. Hollands, unpubl. data; Barembruch and
Hengge, 2007). In the DfliA and Dcrp backgrounds
Activation of s28-dependent transcription by CRP 1099
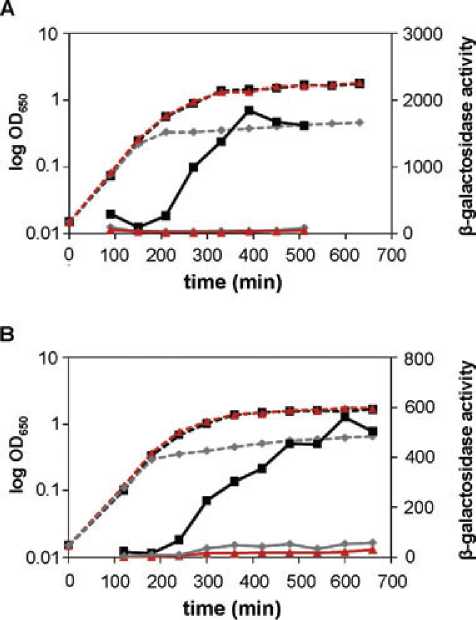
Fig. 1. CRP and s28 dependency of aer promoter activity
throughout growth.
A. Requirement for CRP and s28 in a strain expressing s28 from the
chromosomal fliA promoter. The figure shows OD650 values (dashed
lines) and b-galactosidase activities (in Miller units; solid lines)
measured throughout growth in Escherichia coli K-12 strain M182
(black lines), M182 Dcrp (grey lines) or M182 DfliA (red lines), each
carrying the aer200::lacZ fusion cloned in pRW50.
B. Requirement for CRP and s28 in a strain expressing s28 from a
CRP-independent promoter on plasmid pKXH100. The figure
shows OD650 values (dashed lines) and b-galactosidase activities
(in Miller units; solid lines) measured in strain M182 DfliA containing
pKXH100 (CRP+ FliA+ black lines), strain M182 DfliA Dcrp
containing pKXH100 (CRP- FliA+ grey lines) or strain M182 DfliA
containing ‘empty’ pET21a (CRP+ FliA-; red lines), each carrying
the aer200::lacZ fusion cloned in pRW50.
(Fig. 1A, red and grey lines), promoter activity remained at
a basal level throughout the growth cycle. This confirms
that both CRP and s28 are essential for expression from the
aer regulatory region in vivo. However, this experiment is
complicated by the fact that s28 expression is dependent on
CRP. This is because CRP is required to activate transcrip-
tion of the flhDC operon that encodes an essential activator
of transcription from the fliA promoter (Soutourina et al.,
1999). Indeed, Western blot analysis confirms that no s28
protein is present in strain M182 Dcrp (Fig. S1, lanes 1-3).
To investigate the action of CRP at the aer regulatory
region, independent of the indirect effect of CRP on s28
levels, we established an experimental system in which
expression of s28 is uncoupled from CRP. To do this, we
used DfliA derivatives of M182 and M182 Dcrp that had
© 2009 The Authors
Journal compilation © 2009 Blackwell Publishing Ltd, Molecular Microbiology, 75, 1098-1111
More intriguing information
1. Testing the Information Matrix Equality with Robust Estimators2. The name is absent
3. Strategic Investment and Market Integration
4. The Impact of Individual Investment Behavior for Retirement Welfare: Evidence from the United States and Germany
5. Two-Part Tax Controls for Forest Density and Rotation Time
6. Top-Down Mass Analysis of Protein Tyrosine Nitration: Comparison of Electron Capture Dissociation with “Slow-Heating” Tandem Mass Spectrometry Methods
7. SAEA EDITOR'S REPORT, FEBRUARY 1988
8. Structural Breakpoints in Volatility in International Markets
9. Who’s afraid of critical race theory in education? a reply to Mike Cole’s ‘The color-line and the class struggle’
10. Examining the Regional Aspect of Foreign Direct Investment to Developing Countries