1100 K. Hollands, D. J. Lee, G. S. LloydandS. J. W. Busby ■
В
-зо
GAA TΓCATCAAGGAATTAGATTTGTGTTTAAATCGCA
-€0 -40
Aattgcgatctaaatcaaattaatcggttaaagataa
Aaatgtgatctagatcacattt taaagttt
CRP site <35 element
+1
-20 . J÷ .
CCGCAGC g g Ggccgacataaac ⅛, gac aag aagtt а
Gccgataa
<10element
♦20 b ♦до
ACAAC Catataacct gcас aggac gc Gaacatgtctt
+60
CTCATCCGTATGTCAAGCrT
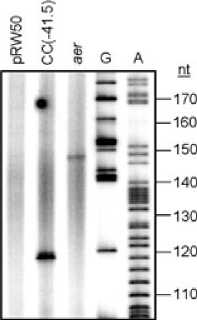
Fig. 2. Identification of the aer transcript start site.
A. The figure shows the result of primer extension analysis using RNA extracted from strain M182, carrying the aer200 fragment cloned in
pRW50, grown aerobically to mid-exponential phase (OD650 0.4-0.6) in LB medium. Control primer extension reactions were also carried out
using RNA extracted from M182 cells containing ‘empty’ pRW50, or pRW50 carrying the CC(-41.5) promoter, whose transcript start site is
known, and which gives a primer extension product of 118 nt in pRW50. The sizes of primer extension products were determined by
calibration against sequencing reactions (lanes G and A).
B. The figure shows the base sequence of the non-template strand of the aer200 promoter fragment used in this work. The transcript start site
proposed here is boxed, and the aer translation start codon is underlined. The proposed -10 and -35 octamer elements of the s28-dependent
aer promoter, and the DNA site for CRP, are highlighted in bold. The consensus sequence for each DNA element is indicated in grey below
the sequence (Busby and Ebright, 1999; Koo et al., 2009). Bases in the -10 and -35 elements that were targeted for mutational analysis are
underlined in bold. The EcoRI and HindIII sites flanking the aer200 fragment are shown in italics, and the sequence is numbered with the aer
transcript start site as +1.
been transformed with pKXH100, which encodes fliA
under the control of a CRP-independent promoter.
Western blot analysis confirms that, in this system,
expression of s28 does not require CRP (Fig. S1, lanes
4-6). We measured expression of the aer200::lacZ fusion
in pRW50 in these strains. Data illustrated in Fig. 1B show
that, in M182 DfliA pKXH100, the activity of the
aer200::lacZ fusion follows a similar pattern to that in
strain M182 (which expresses fliA from the chromosome),
except that the increase in promoter activity occurs later in
growth, once the culture begins to enter stationary phase.
This correlates with a delayed increase in s28 protein
levels in this background (K. Hollands, unpubl. results). In
the absence of CRP, promoter activity remains low
throughout the growth cycle, showing that the require-
ment for CRP for expression from the aer regulatory
region is independent of the effect of CRP on s28 levels.
We conclude that CRP must function directly at the aer
regulatory region, and this is consistent with the previous
observation that introducing mutations into the DNA target
for CRP upstream of aer prevents CRP-dependent acti-
vation of the aer200::lacZ fusion (Hollands et al., 2007).
aer is transcribed from a single s28-dependent
promoter in vivo
Although the aer regulatory region has been predicted as
a target for Es28 (Park et al., 2001; Frye et al., 2006; Zhao
et al., 2007), the promoter determinants required for tran-
scription initiation have not been identified experimentally.
To define the DNA elements required for s28-dependent
transcription of aer, we began by mapping the aer tran-
script start site by primer extension analysis, using the
aer200 promoter fragment cloned in pRW50. This yielded
a single extension product approximately 148 nucleotides
in length (Fig. 2A), which places the transcript start point
at the position labelled +1 in Fig. 2B. This falls 6 bp down-
stream from the -10 octamer element for a s28-dependent
promoter predicted by Park et al. (2001). To examine the
importance of this promoter, we constructed derivatives of
the aer200 fragment containing point mutations in the
putative -10 and -35 elements (Table 1 and Fig. 2B). In
the -10 element, we targeted the highly conserved
5'-CGA-3' motif, from positions -11 to -9, because muta-
tions in this motif result in a loss of s28-dependent tran-
scription from other s28-dependent promoters, both in vivo
and in vitro (Yu et al., 2006; Wozniak and Hughes, 2008).
In the -35 octamer, we targeted positions -32T and -30A,
which are also highly conserved and are important for
s28-dependent transcription from the Salmonella flgM pro-
moter, and position -28, which has more minor effects on
flgM promoter activity (Wozniak and Hughes, 2008). Each
mutant promoter fragment was cloned into pRW50, and
expression of the resulting promoter::lacZ fusions was
measured in the CRP+ FliA+, CRP- FliA+ and CRP+ FliA-
backgrounds. Results listed in Table 1 show that the sub-
© 2009 The Authors
Journal compilation © 2009 Blackwell Publishing Ltd, Molecular Microbiology, 75, 1098-1111
More intriguing information
1. The name is absent2. Globalization, Divergence and Stagnation
3. The name is absent
4. Wirkung einer Feiertagsbereinigung des Länderfinanzausgleichs: eine empirische Analyse des deutschen Finanzausgleichs
5. Spectral calibration of exponential Lévy Models [1]
6. How does an infant acquire the ability of joint attention?: A Constructive Approach
7. The name is absent
8. Synchronisation and Differentiation: Two Stages of Coordinative Structure
9. The name is absent
10. Learning and Endogenous Business Cycles in a Standard Growth Model