Sponsored Document Sponsored Document Sponsored Document
Veerapen et al.
Page 3
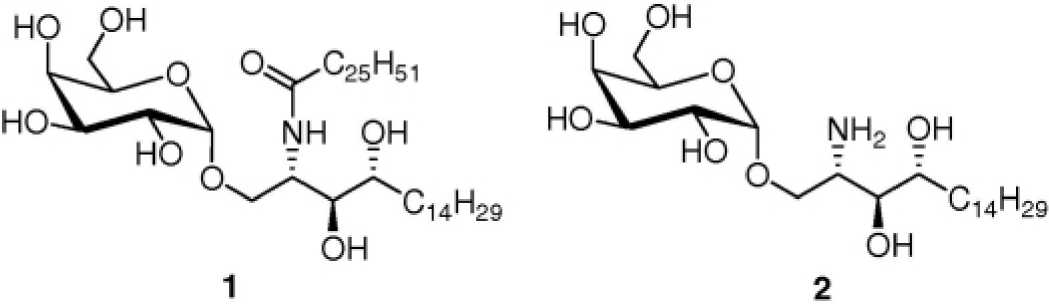
Several elegant syntheses of α-GalCer are present in the literature. Most of these syntheses
make use of benzyl ether protecting groups on the sugar moiety. We, along with many other
research groups, have encountered difficulties in the hydrogenolysis of benzyl ethers, thereby
leading to low yields of α-GalCer. Therefore, synthetic routes circumventing the problematic
hydrogenolysis step are highly desired. Kiso and co-workers recently reported such a synthesis,
where interestingly they also made use of the bulky 4,6-O-di-tert-butylsilylene (DTBS) group
as α-directing in galactosylation donor 3.
Published as: BioorgMed Chem Lett. 2009 August 01; 19(15): 4288-4291.
More intriguing information
1. The name is absent2. Evolutionary Clustering in Indonesian Ethnic Textile Motifs
3. PROTECTING CONTRACT GROWERS OF BROILER CHICKEN INDUSTRY
4. Transport system as an element of sustainable economic growth in the tourist region
5. Tissue Tracking Imaging for Identifying the Origin of Idiopathic Ventricular Arrhythmias: A New Role of Cardiac Ultrasound in Electrophysiology
6. The Complexity Era in Economics
7. Une nouvelle vision de l'économie (The knowledge society: a new approach of the economy)
8. Prizes and Patents: Using Market Signals to Provide Incentives for Innovations
9. The name is absent
10. Update to a program for saving a model fit as a dataset