Sponsored Document Sponsored Document Sponsored Document
Veerapen et al.
Page 4
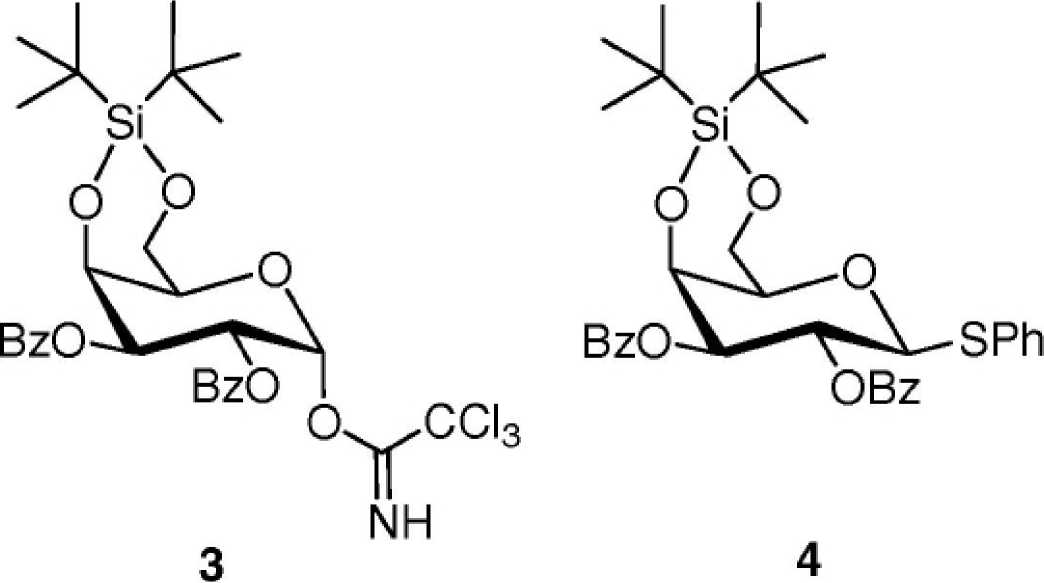
We have employed two different synthetic strategies, including that of Kiso and co-workers
to obtain the glycosylated sphingoid base template 2, which can readily be converted to α-
GalCer and other derivatives. Initially, to improve on the reported synthesis we resorted to the
use of the thioglycosyl donor 4 as it is easier to handle and has a better shelf life than the
trichloroacetimidate donor 3. Significantly, the second route describes a novel synthesis of α-
GalCer and has the additional benefit of providing a crucial intermediate, which can be further
modified to yield other biologically active α-GalCer derivatives.
We first synthesized the phytosphingosine acceptor 5 from (2S,3S,4R)-2-azido-1, 3, 4-
octadecanetriol in three steps as described in Scheme 1.
The thioglycoside 4 was obtained in large scale from commercially available β-D-
galactopyranose pentaacetate after standard procedures, as described previously.The critical
glycosylation was then attempted on 2 g of the donor 4. NIS/TfOH activation of thioglycoside
4 (Scheme 2) in anhydrous CH2Cl2 at -78 °C afforded the glycosylated compound 6 in 71%
yield, as the α-anomer exclusively after two hours. Subsequent sequential removal of the silyl
group with TBAF and Zemplen’s deprotection of the benzoate protecting groups produced the
azide intermediate in quantitative yields after purification by flash chromatography. Different
methods for the reduction of the azide group were attempted, including the use hydrogen
sulfide, but the best results were obtained via hydrogenation in methanol. 800 mg of the amine
2 was hence isolated as a white solid.
Scheme 3 illustrates our novel strategy to synthesise α-GalCer and its derivatives. Silicon
tethered intramolecular glycosylation is a particularly attractive method for generating
glycosidic bonds stereoselectively, but not attempted by many research groups due to the
difficulty in handling and relative instability of the silylene-tethered sugar derivative. However,
successful examples have been reported and we were motivated by Bols work on the synthesis
Published as: BioorgMed Chem Lett. 2009 August 01; 19(15): 4288-4291.
More intriguing information
1. Non Linear Contracting and Endogenous Buyer Power between Manufacturers and Retailers: Empirical Evidence on Food Retailing in France2. TRADE NEGOTIATIONS AND THE FUTURE OF AMERICAN AGRICULTURE
3. Sex differences in the structure and stability of children’s playground social networks and their overlap with friendship relations
4. The name is absent
5. Land Police in Mozambique: Future Perspectives
6. Financial Markets and International Risk Sharing
7. The name is absent
8. Can genetic algorithms explain experimental anomalies? An application to common property resources
9. Confusion and Reinforcement Learning in Experimental Public Goods Games
10. Social Balance Theory