(b)
SAFT
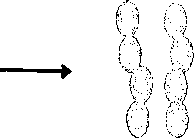
Chainsmadeof
mSEGMENTS
σ
REFERENCE FLUID
Ofhardspheres
SAFT - D
σ
REFERENCE FLUID DIMER FLUID CHAINS MADE OF
Ofhardspheres m segments
Figure 2.3: Schematic illustration of the differences between the SAFT and SAFT-D
approaches.
2.2.2 Dispersion term
Now that the equation of state for hard chains is defined, the contribution that
will account for the dispersive interactions between molecules is to be added. The
model potential used to describe the attraction is given by the square-well potential.
(2.19)
where A is the width of the potential well, A = 1.5σ. An accurate expression for this
attraction∕dispersion contribution to the free energy was developed by Gross and
Sadowski [48] based on the extension of the perturbation theory of Barker and Hen-
44
More intriguing information
1. SLA RESEARCH ON SELF-DIRECTION: THEORETICAL AND PRACTICAL ISSUES2. The name is absent
3. WP 1 - The first part-time economy in the world. Does it work?
4. he Effect of Phosphorylation on the Electron Capture Dissociation of Peptide Ions
5. Reversal of Fortune: Macroeconomic Policy, International Finance, and Banking in Japan
6. Who runs the IFIs?
7. Testing Panel Data Regression Models with Spatial Error Correlation
8. On the job rotation problem
9. Analyse des verbraucherorientierten Qualitätsurteils mittels assoziativer Verfahren am Beispiel von Schweinefleisch und Kartoffeln
10. Cardiac Arrhythmia and Geomagnetic Activity