Since almost half of the survey population in our study would have behaved differently if they were
personally affected, we suggest that altruism plays a role in WTP valuations.
4.2 Testing for the ambiguity effect
The statistical tests conducted to examine the ambiguity effects are presented in Table 4. The first
test we conducted was to examine the null hypothesis in treatment 3 that the bid distribution in the 5th trial
between the group given the unambiguous incidence rate and the group that was not given the
unambiguous incidence rate is identical using Wilcoxon’s signed-rank test. The test result indicates that
there is no difference statistically (p=0.436). Hence, we found no significant ambiguity effects in the
within-sample design of the experiment. This result is confirmed by the summary statistics in Table 3 as
well. Clearly, the information about the unambiguous incidence rate did not significantly affect the level
of the bids in treatment 3. However, comparing the fifth trial before the unambiguous incidence rate was
mentioned and the first trial after the unambiguous incidence rate was mentioned, the difference between
the mean WTP is statistically different at the 5% level (p=0.013). The unambiguous incidence rate
information clearly decreased the mean WTP significantly from 129 to 87 Eurocents (see Figure 2). This
can be interpreted as being an ambiguity effect since the new information about the unambiguous
incidence rate significantly diminished the WTP in the trial right after the unambiguous information was
provided. However, it is not clear why the bids in subsequent trials increased.
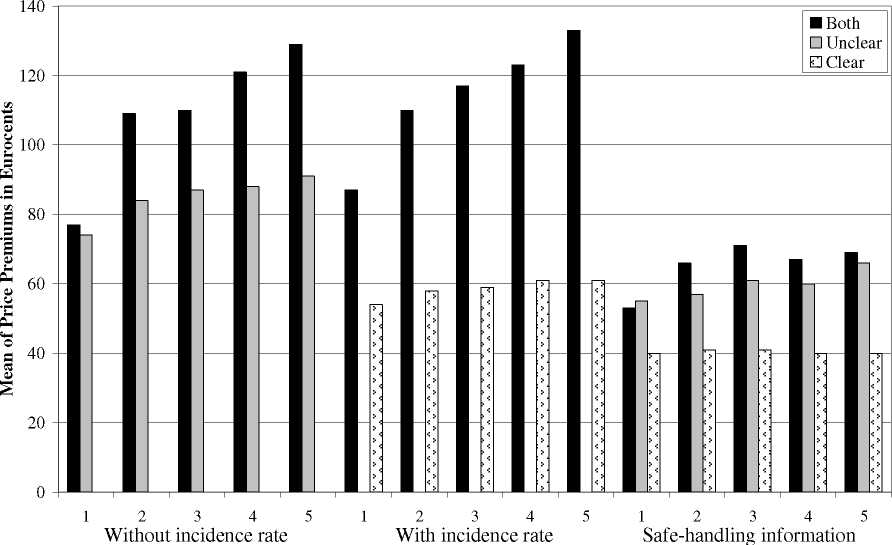
Figure 2. Comparison of trials for the different treatments
Using the Kolmogorov-Smirnov two-sample test, we tested the null hypothesis that the population
distributions of the bids between the different treatments are identical. We tested if treatment 1 bids differ
from treatment 3 bids before provision of the unambiguous incidence rate information. The null
hypothesis cannot be rejected (p=0.303) suggesting that we could not find ambiguity effects between the
two treatments. We also tested if treatment 2 bids differ from treatment 3 bids after the provision of the
unambiguous incidence rate information. The null hypothesis of this test also cannot be rejected.
More intriguing information
1. THE DIGITAL DIVIDE: COMPUTER USE, BASIC SKILLS AND EMPLOYMENT2. Macroeconomic Interdependence in a Two-Country DSGE Model under Diverging Interest-Rate Rules
3. The Composition of Government Spending and the Real Exchange Rate
4. On the Real Exchange Rate Effects of Higher Electricity Prices in South Africa
5. Developmental changes in the theta response system: a single sweep analysis
6. Delayed Manifestation of T ransurethral Syndrome as a Complication of T ransurethral Prostatic Resection
7. The name is absent
8. Determinants of U.S. Textile and Apparel Import Trade
9. Detecting Multiple Breaks in Financial Market Volatility Dynamics
10. Does Competition Increase Economic Efficiency in Swedish County Councils?