304 I J BUJALSKA and others . 11b-HSD1 is essential for human adipogenesis
C FABP4
11β-HSD1
Ctr PF-877423 E E+PF-877423
Ctr PF-877423
E E+PF-877423
D G3PD
E Ctr
E E+PF-877423 F
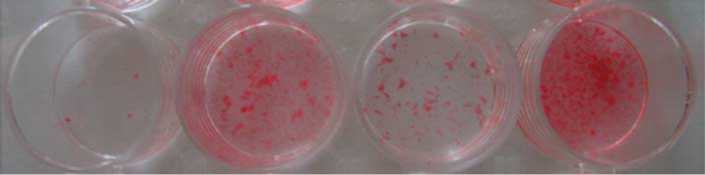
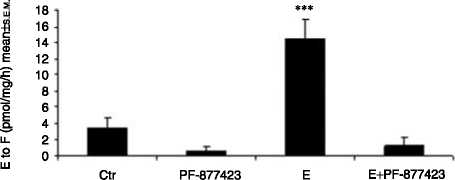
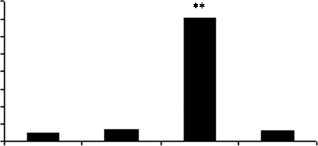
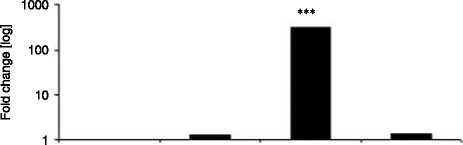
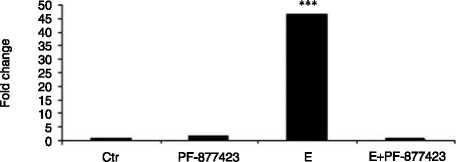
Figure 6 Chub-S7 cells differentiated with 0. 5 mM E and with or without the selective 11b-HSD1 inhibitor PF-877423 at 100 nM. All
experiments were carried out on differentiated cells on day 10, nZ3. (A) 11b-HSD1 oxo-reductase activity (cortisone to cortisol
conversion): 14.6G2.4 (E) versus 1.2G1.1 (ECPF-877423) vs 3.4G1.3 (control), pmol/mg per h meanGS.E.M., P!0.001, (B) 11b-HSD1
mRNA expression and adipogenic markers (C) FABP4, (D) G3PD (log scale, P!0.001 and P!0.001 respectively) and (E) Chub-S7 cells
differentiated for 21 days and stained with oil red O; 1) control: 166 nM insulin, PPARg agonist, 100 nM PF-877423, 2) 0.5 mME,
3) 0.5 mM EC100 nM PF-877423 and 4) positive control (differentiation with 0.5 mM F). P values: **P!0.01, ***P!0.001.
PF-877423 selectivity was undertaken using a transformed
cell line expressing human 11b-HSD2 enzyme where no
inhibitory effect was observed. Incubation of cells with 100 nM
PF-877423 completely abolished the cortisone induction of
FABP4, G3PDH and 11b-HSD1 itself in Chubb-S7 cells. We
also demonstrated that the transformed cell line data were
consistent with data in primary human subcutaneous pre-
adipocytes. While incubation with cortisone yielded a less
impressive effect on adipogenesis in human subcutaneous
preadipocytes when compared with Chubb-S7 cells (this
might reflect a more advanced adipogenic lineage of primary
cultures), inhibition of 11b-HSD1 activity nevertheless reduced
the ability of human subcutaneous preadipocytes to differentiate
and accumulate lipid.
Whilst the cell line that we have used is subcutaneous in
origin and the expression of 11b-HSD1 is higher in omental
human preadipocytes (Bujalska et al. 1997b) and therefore we
predict that the impact upon omental cells would be more
pronounced. We anticipate that this would not lead to
preferential loss of subcutaneous fat. Unfortunately, omental
cell lines are not available for study and in vivo human clinical
studies have not been performed.
Previously, non-selective 11b-HSD inhibitors have been
shown to diminish human adipocyte differentiation in vitro
(Bujalska et al. 1999) and increase insulin sensitivity in man
(Walker et al. 1995), but a lack of isozyme selectivity can cause
water retention and hypertension. Since then, patents have been
filed on compounds that report to be selective 11b-HSD1
inhibitors. An aryl sulphonamide derivative has been shown to
reduce insulin levels and improve glucose tolerance when
administered to rodents for 7 days (Alberts et al. 2002). Similar
data have been reported for an adamantyl triazole that also
reduced body weight and the progression of atherosclerosis in
mice (Hermanowski-Vosatka et al. 2005). Transgenic mouse
models have established 11b-HSD1 as a novel therapeutic target
in this regard; global deletion of 11b-HSD1 results in improved
Journal of Endocrinology (2008) 197, 297-307
www.endocrinology-journals.org
More intriguing information
1. Update to a program for saving a model fit as a dataset2. The name is absent
3. The name is absent
4. The name is absent
5. Electricity output in Spain: Economic analysis of the activity after liberalization
6. Asymmetric transfer of the dynamic motion aftereffect between first- and second-order cues and among different second-order cues
7. BUSINESS SUCCESS: WHAT FACTORS REALLY MATTER?
8. The name is absent
9. Detecting Multiple Breaks in Financial Market Volatility Dynamics
10. A Pure Test for the Elasticity of Yield Spreads