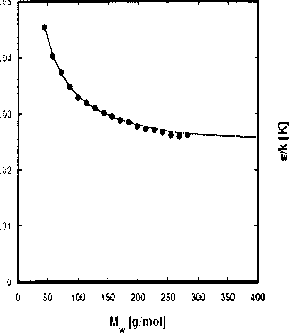
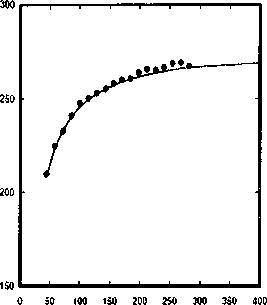
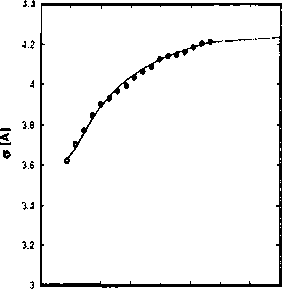
Figure 2.6: Pure component parameters for the n-alkane series as a function of the molar
mass.
Mw [g'moɪ]
0 SO ICO (SO 200 250 300 3S0 400
Mw [g'mo∣∣
where parameter(k) is one of {r∏i∕Mwi,σi,ti∕k'). The coefficients of the correlations
are given in table 2.3. The correlations are also plotted in figure 2.6. These correla-
tions are used to extrapolate the parameters for higher alkanes.
|
parameter (k) |
units |
qp⅛ |
q⅛ |
qafc |
|
m∕Mu, |
mol∕g |
3.626670923 |
0.44366558 |
0.373239091 |
|
σ |
 |
0.045349657 |
-0.021681925 |
-0.001005353 |
|
e∕k |
K |
209.4657254 |
64.23483985 |
5.152948312 |
Table 2.3: Constants used in the correlation presented in Eq. (2.31) for the dependence of
SAFT-D parameters on molecular weight of n-alkanes.
Figure 2.7 compares experimental saturated liquid densities of tetracosane(n-
C24), triacontane(n-C30), and hexatriacontane(n-C36), with those calculated from
PC-SAFT and the new equation of state using extrapolated parameters. The new
EOS performs better than PC-SAFT and give reasonably accurate densities for all
three components in the range of temperature considered.
52
More intriguing information
1. Handling the measurement error problem by means of panel data: Moment methods applied on firm data2. The Mathematical Components of Engineering
3. EDUCATIONAL ACTIVITIES IN TENNESSEE ON WATER USE AND CONTROL - AGRICULTURAL PHASES
4. The name is absent
5. sycnoιogιcaι spaces
6. The name is absent
7. The name is absent
8. The name is absent
9. Technological progress, organizational change and the size of the Human Resources Department
10. How do investors' expectations drive asset prices?