Chapter 4
than with Ca/ Mg ions. The reason may be the adsorptions of Ca/ Mg ions on the
negatively charged surface sites of kaolinite. When Ca/ Mg ions adsorb on the
surface of kaolinite, the negatively-charged sites will become positively- charged.
Thus the net surface charge of kaolinite will become less negative. In the figure,
zeta potential will becomes more negative when adding sodium hydroxide,
sodium silicate, sodium citrate or sodium carbonate.
-10
-15
-55
O
O
-20
-3
w
-40
-45,
-50
ω
O
S
φ
N
-30
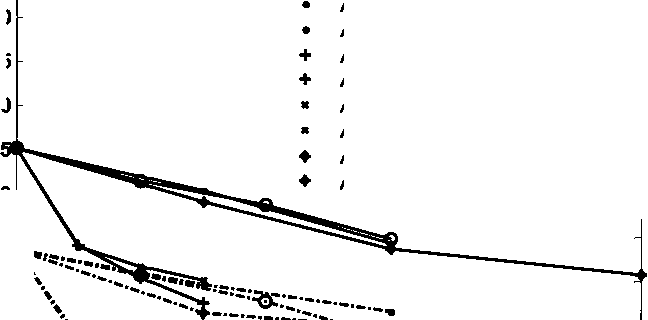
Adding NaOH, with Ca/Mg
Adding NaOH, without Ca/Mg
Adding carbonate, with Ca/Mg
Adding carbonate, without Ca/Mg
Adding metasilicate, with Ca/Mg
Adding metasilicate, without Ca/Mg
Adding orthosilicate, with Ca/Mg
Adding orthosilicate, without Ca/Mg
Adding citrate, with Ca/Mg
Adding citrate, without Ca/Mg
*''∙t:
-60
-65 l
0
0.2
0.4
0.6
0.8
Concentration (mM)
Figure 4.16 Zeta potentials of kaolinite in synthetic brine with different additives
Figure4.17 shows the zeta potential change (mV∕mM) as the function of
additive concentration. Central difference method is used for calculation.
121
More intriguing information
1. Cardiac Arrhythmia and Geomagnetic Activity2. Examining Variations of Prominent Features in Genre Classification
3. The name is absent
4. The name is absent
5. Delivering job search services in rural labour markets: the role of ICT
6. Investment in Next Generation Networks and the Role of Regulation: A Real Option Approach
7. The name is absent
8. Olfactory Neuroblastoma: Diagnostic Difficulty
9. The name is absent
10. Developments and Development Directions of Electronic Trade Platforms in US and European Agri-Food Markets: Impact on Sector Organization