Chapter 4
values are 2.86×10^8 mol∕m3, 3.33×10^1° mol∕m3 and 1.85×10^7 mol∕m2,
respectively. For amphoteric alumina sites, kaolinite has the same magnitude of
dissociation equilibrium constants and site density as alumina. This indicates
kaolinite has similar amphoteric alumina sites to alumina and validates the
assumption in section 4.3.2.
Table 4.9 Alumina site densities and dissociation constants
|
Reaction |
K (mol∕m3) |
Λ∕( W8 mol∕m2) |
|
-aioh; -aioh+h+ |
4.21×10^8 |
10.9 |
|
-AlOH -AlO +H+ |
3.58×10^1° |
1
0.9
0.8
0.7
O 0.6
о
≡ 0.5
U)
±s 0.4
V)
0.3
0.2
0.1
0
6 7 8 9 10 11
Bulk pH
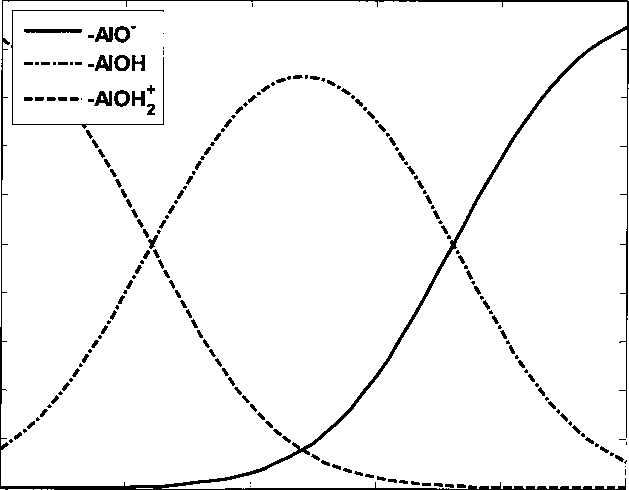
Figure 4.22 Alumina sites fraction at different bulk pH in 0.05 M NaCI
129
More intriguing information
1. Income Taxation when Markets are Incomplete2. Insecure Property Rights and Growth: The Roles of Appropriation Costs, Wealth Effects, and Heterogeneity
3. THE WAEA -- WHICH NICHE IN THE PROFESSION?
4. Feature type effects in semantic memory: An event related potentials study
5. EMU's Decentralized System of Fiscal Policy
6. The Shepherd Sinfonia
7. Bridging Micro- and Macro-Analyses of the EU Sugar Program: Methods and Insights
8. Announcement effects of convertible bond loans versus warrant-bond loans: An empirical analysis for the Dutch market
9. Structural Breakpoints in Volatility in International Markets
10. Measuring Semantic Similarity by Latent Relational Analysis