Chapter 4
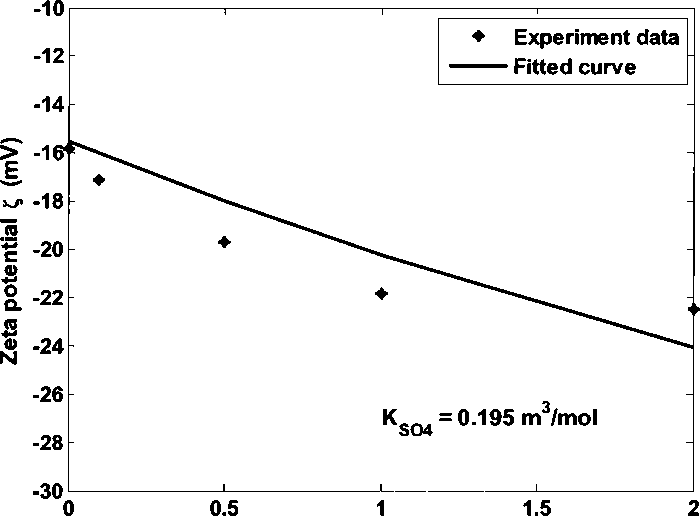
kaolinite as the function of Na2SO4 concentration in 0.05 M NaCI brine at pH 6.5.
The adsorption constant ∕<s04 is 0.195 m3∕mol.
C(Na2SO4) (M)
x103
Figure 4.23 Kaolinite zeta potential in 0.05 M NaCI brine adding Na2SO4 at pH 6.5
In order to study the effect of bicarbonate, zeta potentials of kaolinite with
different NaHCO3 in de-ionized water at pH 8.3 were measured. In NaHCO3
solution, positively charged sites -AOH2+ can adsorb HCO3^ ion and become
neutral sites -AOH2HCO3.
-AOH2 +HCO3 f≡ -AOH2HCO3 [4.34]
κ _ [AOH2HCO3] [AOH2HCO3]
hc°3 [AOH2]α∕HCO3) [AQH2 ]αz, (HCO3 )exp(-)
131
More intriguing information
1. Human Development and Regional Disparities in Iran:A Policy Model2. Electricity output in Spain: Economic analysis of the activity after liberalization
3. Text of a letter
4. Om Økonomi, matematik og videnskabelighed - et bud på provokation
5. The name is absent
6. The name is absent
7. The name is absent
8. On the Desirability of Taxing Charitable Contributions
9. PRIORITIES IN THE CHANGING WORLD OF AGRICULTURE
10. Housing Market in Malaga: An Application of the Hedonic Methodology