Chapter 4
M NaCl brine adding CaCI2 or MgCI2 at pH 6.5 were measured.
-AO' +Ca2+ z≠ -AOCa+, -AO +Mg2+ -AOMg+ [4.37]
= [ AOCa+] = [ AOCa+]
'^ [A0]α,(Ca2*) [Aθ]a√Ca')exp(-¾
kι
= [AOMg+] = [A0Mg+]
M8 [a0^MmS2+) [AO⅛(Mg2+)exp(-¾
kT
σ0(Ca) = e([AOH+]-[AO']-[B']+[AOCa+]) [4.40]
σ0 (Mg) = e([AOH+] - [AO ] - [B' ]+[A0Mg+ ]) [4.41 ]
Here Kca and Kwg are surface adsorption equilibrium constants.
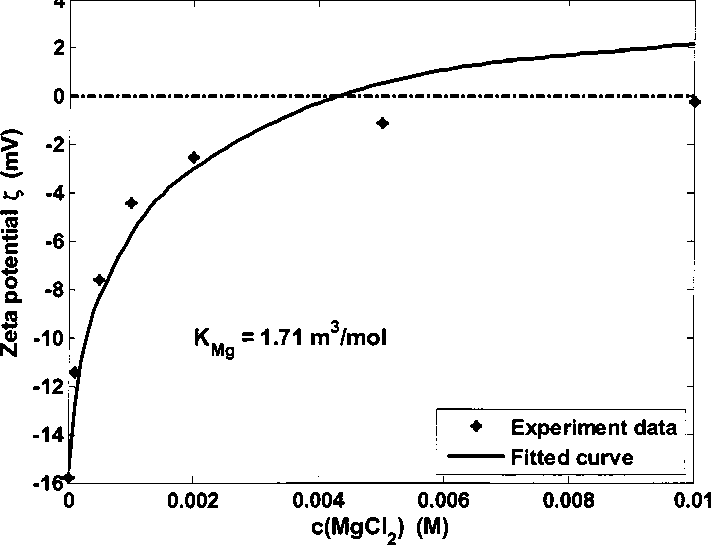
Figure 4.26 Kaolinite zeta potential in 0.05 M NaCI brine adding MgCI2 at pH 6.5
134
More intriguing information
1. The name is absent2. The name is absent
3. Fighting windmills? EU industrial interests and global climate negotiations
4. An Investigation of transience upon mothers of primary-aged children and their school
5. DURABLE CONSUMPTION AS A STATUS GOOD: A STUDY OF NEOCLASSICAL CASES
6. Evolution of cognitive function via redeployment of brain areas
7. Wounds and reinscriptions: schools, sexualities and performative subjects
8. Errors in recorded security prices and the turn-of-the year effect
9. The name is absent
10. Empirically Analyzing the Impacts of U.S. Export Credit Programs on U.S. Agricultural Export Competitiveness