Chapter 4
assuming НСОз’ ion adsorption is closer to the experiment data than the curve
without assuming НСОз’ ion adsorption.
From zeta potential result, anions SO42’ or HCO3^ can adsorb on positively
charged surface sites and make kaolinite zeta potential more negative.
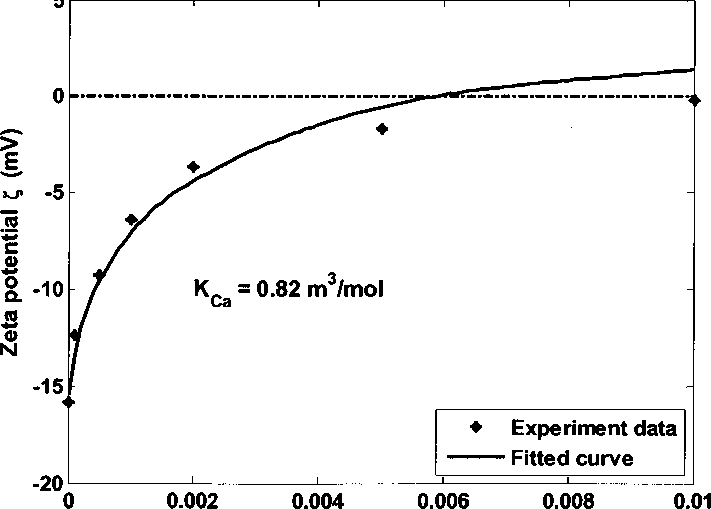
4.3.5.3. Adsorption effect of cations Ca2+ and Mg2+
Synthetic brine contains cations Ca2+ and Mg2+. The negatively charged Al/
Si sites -AO’ can adsorb Ca/ Mg ions and become positively charged.
C(CaCl2) (M)
Figure 4.25 Kaolinite zeta potential in 0.05 M NaCI brine adding CaCI2 at pH 6.5
In order to study the effect of Ca/ Mg ions, zeta potentials of kaolinite in 0.05
133
More intriguing information
1. Labour Market Institutions and the Personal Distribution of Income in the OECD2. Altruism with Social Roots: An Emerging Literature
3. The name is absent
4. Implementation of Rule Based Algorithm for Sandhi-Vicheda Of Compound Hindi Words
5. THE DIGITAL DIVIDE: COMPUTER USE, BASIC SKILLS AND EMPLOYMENT
6. The name is absent
7. ‘I’m so much more myself now, coming back to work’ - working class mothers, paid work and childcare.
8. The name is absent
9. The Role of Immigration in Sustaining the Social Security System: A Political Economy Approach
10. The name is absent