Chapter 4
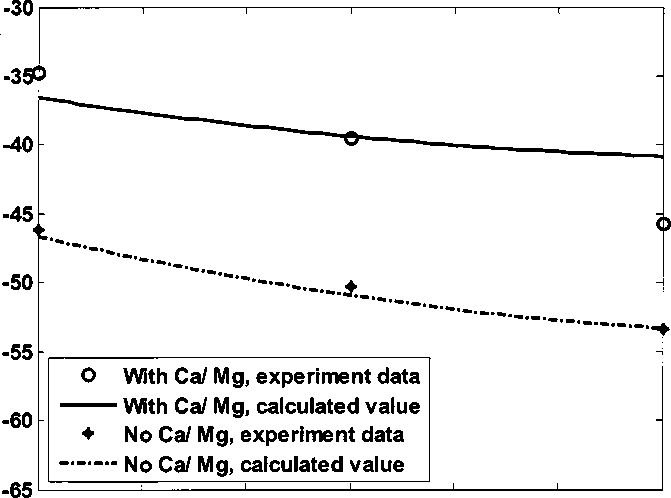
Figure 4.28 shows zeta potential of kaolinite in synthetic brine with or without
Ca/ Mg adding Na2CO3. Adding 6.0×10'4 M Na2CO3, pH of the brine increases
from 8.3 to 8.9. The blue dashed curve shows the calculated zeta potential of
kaolinite without Ca/ Mg. The red solid curve shows the calculated zeta potential
of kaolinite with Ca/ Mg, with equilibrium Ca2+ concentration calculated from
CaCO3 solubility product.
0 1 2 3 4 5 6
C (Na2CO3) (M)
x1θ"t
Figure 4.28 Kaolinite zeta potential in synthetic brine adding Na2CO3, pH 8.3 - 8.9
In the brine without Ca/ Mg, zeta potential change of kaolinite is around 15
mV∕mM. In the brine with Ca/ Mg, zeta potential change of kaolinite is around 20
mV∕mM. The precipitation of Ca2+ ion can make kaolinite zeta potential more
137
More intriguing information
1. The name is absent2. Quelles politiques de développement durable au Mali et à Madagascar ?
3. The Role of State Trading Enterprises and Their Impact on Agricultural Development and Economic Growth in Developing Countries
4. The name is absent
5. The name is absent
6. Who runs the IFIs?
7. APPLYING BIOSOLIDS: ISSUES FOR VIRGINIA AGRICULTURE
8. The name is absent
9. The name is absent
10. Enterpreneurship and problems of specialists training in Ukraine