Chapter 4
wettability of kaolinite from water-wet to more oil-wet. Kaolinite is partially
positively charged. The positively-charged sites can adsorb anionic naphthenate
and become oil-wet.
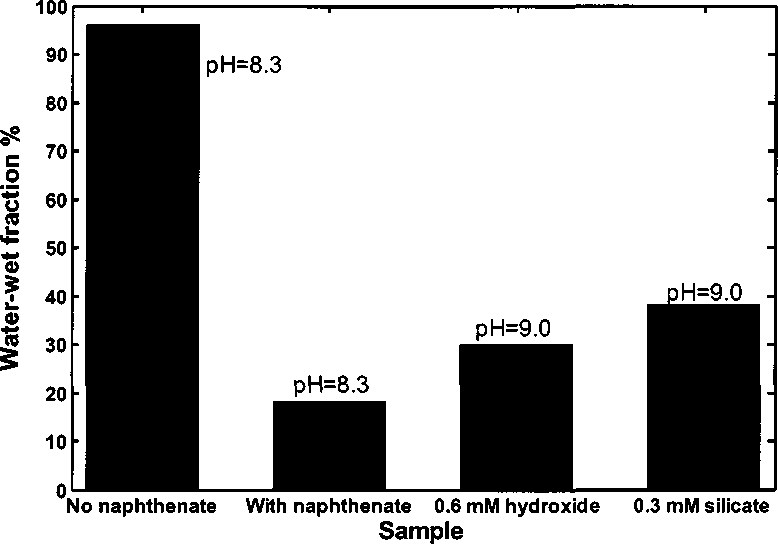
Figure 4.3 Water-wet fraction of kaolinite in toluene-brine mixture with 1.0 %
kaolinite
The third bar is water-wet fraction (30%) in the sample with 100 ppm sodium
naphthenate adding 0.6 mM NaOH at pH 9.0. Compared with the second bar,
water-wet fraction increases. Adding NaOH can convert some of the positively-
charged sites to negatively-charged sites. This can reduce the adsorption of
naphthenate on kaolinite surface and make kaolinite more water-wet.
The last bar is water-wet fraction (38%) in the sample with 100 ppm sodium
93
More intriguing information
1. The name is absent2. The name is absent
3. The name is absent
4. Developments and Development Directions of Electronic Trade Platforms in US and European Agri-Food Markets: Impact on Sector Organization
5. A dynamic approach to the tendency of industries to cluster
6. Putting Globalization and Concentration in the Agri-food Sector into Context
7. Estimated Open Economy New Keynesian Phillips Curves for the G7
8. Heterogeneity of Investors and Asset Pricing in a Risk-Value World
9. CURRENT CHALLENGES FOR AGRICULTURAL POLICY
10. Contribution of Economics to Design of Sustainable Cattle Breeding Programs in Eastern Africa: A Choice Experiment Approach