Chapter 4
significant at higher pH and kaolinite is more water-wet. At the same pH, adding
silicate can make kaolinite more water-wet than adding NaOH. NaOH only has
caustic effect to increase pH. For silicate, besides caustic effect, adsorption of
silicate ion on kaolinite positively-charged sites can make the surface of kaolinite
more negatively-charged and make kaolinite become more water-wet.
100
Wettability test
80
60
40
20
Control 24 ppm NaOH 37 ppm silicate 200 ppm NaOH 366 ppm silicate
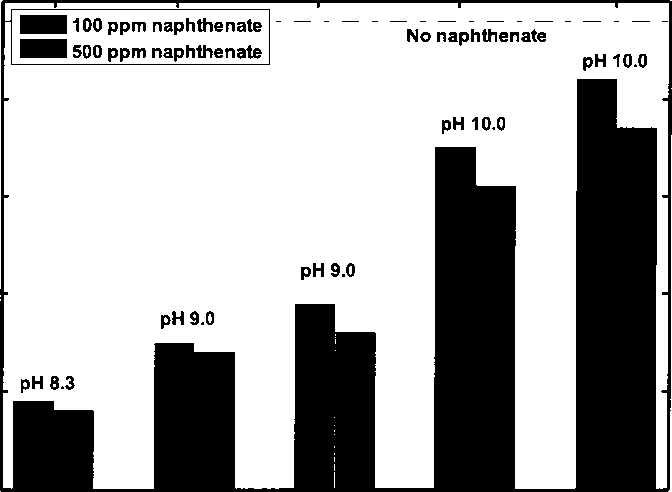
Figure 4.4 Water-wet fraction of kaolinite with different amount of naphthenate
adding NaOH/ Na2SiO3 at different pH
Figure 4.5 shows water-wet fraction of 1.0 w.% kaolinite in toluene-brine
mixture (1:1, v∕v) with 100 ppm naphthenate adding NaOH/ Na2SiO3 at different
pH 24 hours after preparation. Kaolinite becomes more water-wet as pH
95
More intriguing information
1. The name is absent2. Demand Potential for Goat Meat in Southern States: Empirical Evidence from a Multi-State Goat Meat Consumer Survey
3. Opciones de política económica en el Perú 2011-2015
4. Linkages between research, scholarship and teaching in universities in China
5. The migration of unskilled youth: Is there any wage gain?
6. Ability grouping in the secondary school: attitudes of teachers of practically based subjects
7. The Shepherd Sinfonia
8. Quality practices, priorities and performance: an international study
9. The name is absent
10. PEER-REVIEWED FINAL EDITED VERSION OF ARTICLE PRIOR TO PUBLICATION