Chapter 4
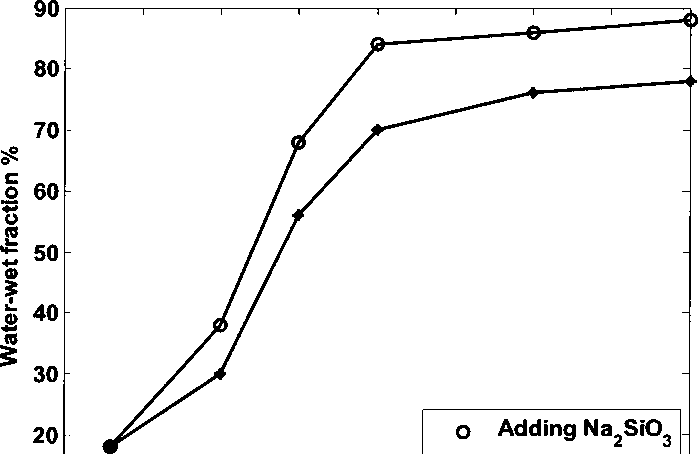
increases. At the same pH, adding silicate can make kaolinite more water-wet
than adding NaOH. Below pH 10.0, water-wet fraction of kaolinite increases
rapidly as pH increases. At pH 10.0, water-wet fractions of kaolinite adding NaOH
or Na2SiO3 are 70% and 84%, respectively. Above pH 10.0, water-wet fraction
increases slowly as pH increases and reaches plateau. Water/ crude oil system
with high pH will form O∕W emulsion spontaneously, which should be avoided in
emulsion separation process. Hence pH 10.0 is upper bound for employing
wettability change using NaOH or Na2SiO3 in separation process.
♦ Adding NaOH
1°8 8.5 9 9.5 10 10.5 11 11.5 12
pH
Figure 4.5 Water-wet fraction of kaolinite with 100 ppm naphthenate adding
NaOH/ Na2SiO3 at different pH
96
More intriguing information
1. Job quality and labour market performance2. Behavioural Characteristics and Financial Distress
3. ENVIRONMENTAL POLICY: THE LEGISLATIVE AND REGULATORY AGENDA
4. WP 48 - Population ageing in the Netherlands: Demographic and financial arguments for a balanced approach
5. The name is absent
6. Trade Liberalization, Firm Performance and Labour Market Outcomes in the Developing World: What Can We Learn from Micro-LevelData?
7. Experience, Innovation and Productivity - Empirical Evidence from Italy's Slowdown
8. The name is absent
9. Importing Feminist Criticism
10. The name is absent