Chapter 4
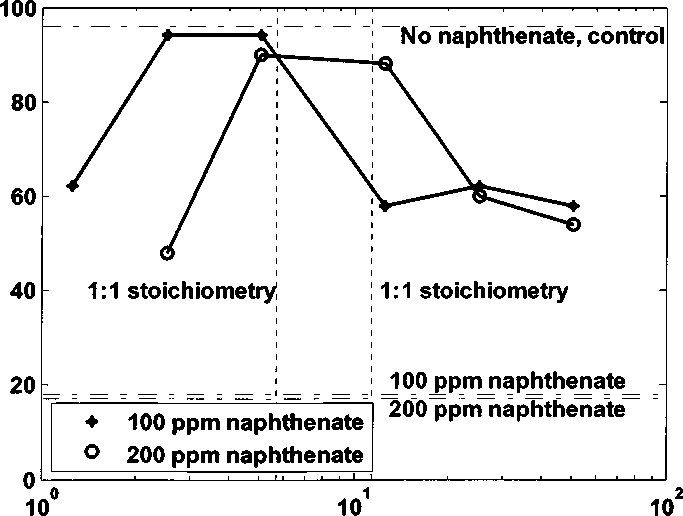
The blue and red dotted lines show 1:1 stoichiometry of CeTAB with 100 ppm /
200 ppm naphthenate (2.25×1 O’514.50×10^5 M). For both cases adding 100 ppm I
200 ppm naphthenate, as concentration of naphthenate increases, water-wet
fraction of kaolinite increases to maximum and then decreases. The optimal
concentration of C8TAB (water-wet fraction of kaolinite reaches maximum) is
close to the 1:1 stoichiometry value (equal molar concentration of C8TAB and
naphthenate) with 100 ppm 1200 ppm naphthenate. If the amount of naphthenate
doubles, the optimal concentration of C8TAB also doubles.
CJAB (ppm)
O
Figure 4.6 Water-wet fraction of kaolinite with 100/ 200 ppm naphthenate adding
different amount of C8TAB at pH 8.3
99
More intriguing information
1. The name is absent2. Population ageing, taxation, pensions and health costs, CHERE Working Paper 2007/10
3. A Hybrid Neural Network and Virtual Reality System for Spatial Language Processing
4. Naïve Bayes vs. Decision Trees vs. Neural Networks in the Classification of Training Web Pages
5. The name is absent
6. The name is absent
7. The name is absent
8. The duration of fixed exchange rate regimes
9. Opciones de política económica en el Perú 2011-2015
10. CHANGING PRICES, CHANGING CIGARETTE CONSUMPTION