Chapter 4
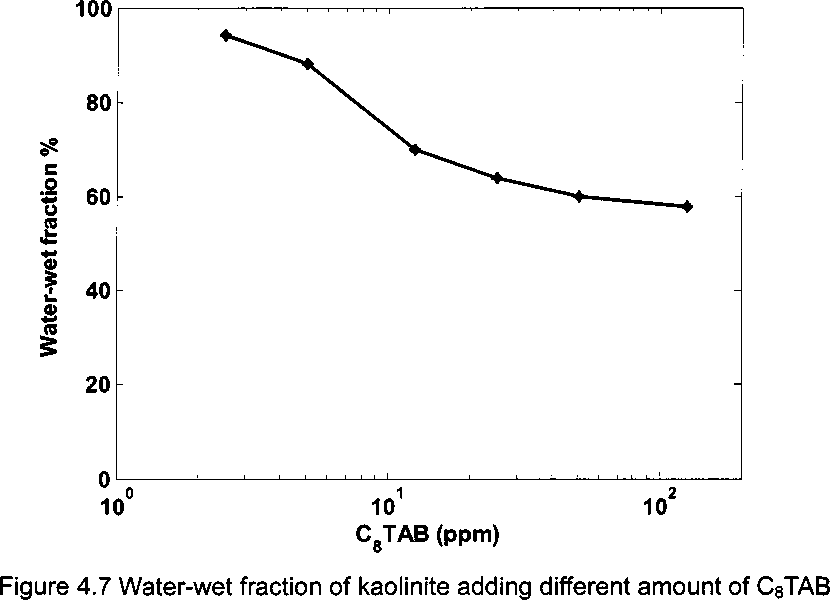
Figure 4.7 shows water-wet fraction of 1.0 w.% kaolinite in toluene-brine
mixture (1:1, v∕v) adding different amounts of CsTAB at pH 8.3 24 hours after
preparation, without naphthenate added. In the figure, water-wet fraction of
kaolinite decreases as CsTAB concentration increases. This reveals that adding
CsTAB only makes kaolinite more oil-wet. Cationic surfactant CsTAB can interact
with negatively-charged groups, for instance, negatively-charged sites on kaolinite
surface or with naphthenate. In the absence of naphthenate, CsTAB will adsorb on
the surfaces of kaolinite with negatively-charged sites and make these surfaces
more oil-wet. Ifthe system contains naphthenate, C8TAB will interact preferentially
100
More intriguing information
1. The economic value of food labels: A lab experiment on safer infant milk formula2. Improving behaviour classification consistency: a technique from biological taxonomy
3. Bargaining Power and Equilibrium Consumption
4. Ventas callejeras y espacio público: efectos sobre el comercio de Bogotá
5. Financial Markets and International Risk Sharing
6. Implementation of the Ordinal Shapley Value for a three-agent economy
7. An Investigation of transience upon mothers of primary-aged children and their school
8. The name is absent
9. The name is absent
10. Correlates of Alcoholic Blackout Experience