Chapter 4
can accept a hydrogen ion and becomes positively charged. The ratio of this type
of cation depends on the association equilibrium constants and pH. At pH 8.3,
only a small amount of betaine becomes positively charged and interacts with
naphthenate to form ion pairs. Hence the optimal dosage is larger than the 1:1
stoichiometry value.
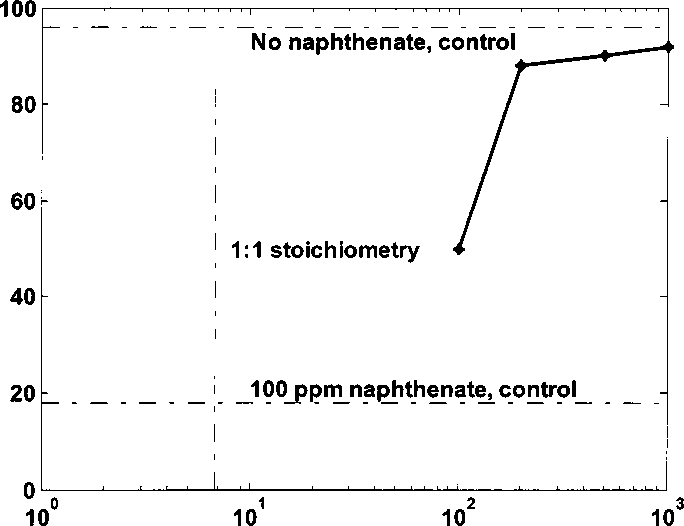
Caprylamidopropyl betaine (ppm)
Figure 4.9 Water-wet fraction of kaolinite with 100 ppm naphthenate adding
different amount of betaine 13 at pH 8.3
Figure 4.10 shows water-wet fraction of 1.0 w.% kaolinite in toluene-brine
mixture (1:1, v∕v) adding different amount of betaine 13 at pH 8.3 24 hours after
preparation, without naphthenate added. Similar to CsTAB, water-wet fraction of
103
More intriguing information
1. Applications of Evolutionary Economic Geography2. The name is absent
3. The name is absent
4. The name is absent
5. The name is absent
6. The name is absent
7. Globalization, Divergence and Stagnation
8. Party Groups and Policy Positions in the European Parliament
9. The name is absent
10. The Role of Land Retirement Programs for Management of Water Resources