Chapter 4
kaolinite decreases as betaine 13 concentration increases. This indicates that
adding betaine 13 only makes kaolinite more oil-wet. The decrease is Slowerthan
CsTAB. Cationic ion Ofbetaine 13 can Interactwith naphthenate. In the absence of
naphthenate, betaine 13 will adsorb on the negative surfaces of kaolinite and
make them more oil-wet. If the system contains naphthenate, cationic ion betaine
13 will interact with naphthenate and form ion pairs, which can make kaolinite
more water-wet.
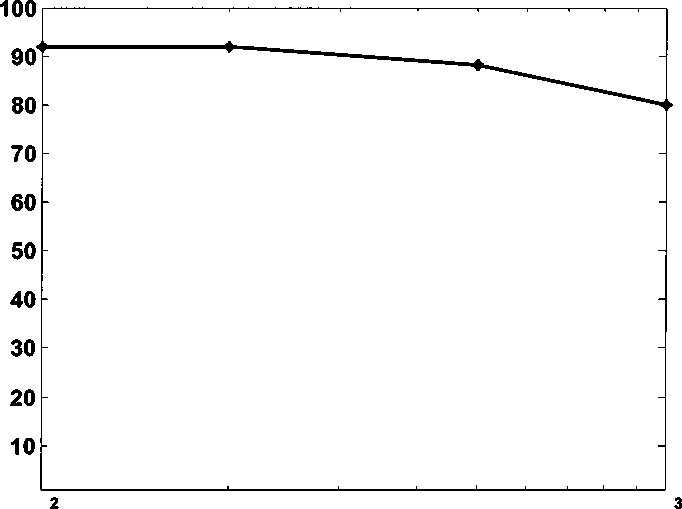
Caprylamidopropyl betaine (ppm)
Figure 4.10 Water-wet fraction of kaolinite adding different amount of betaine 13
at pH 8.3
104
More intriguing information
1. Quality Enhancement for E-Learning Courses: The Role of Student Feedback2. Word Sense Disambiguation by Web Mining for Word Co-occurrence Probabilities
3. The name is absent
4. The name is absent
5. The name is absent
6. PROFITABILITY OF ALFALFA HAY STORAGE USING PROBABILITIES: AN EXTENSION APPROACH
7. The Making of Cultural Policy: A European Perspective
8. The name is absent
9. Dual Track Reforms: With and Without Losers
10. The name is absent